Single-Step Methylation of Chitosan Using Dimethyl Carbonate as a Green Methylating Agent
Abstract
:1. Introduction
- (i)
- (ii)
- stoichiometric processes are involved, thereby cogenerating salts that need to be disposed of;
- (iii)
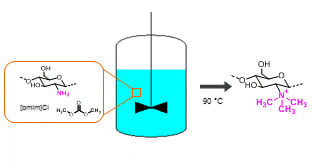
2. Results
3. Materials and Methods
3.1. Methylation of Chitosan Using Dimethyl Carbonate
3.2. Methylation of Chitosan Using Methyl Iodide
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Mourya, V.K.; Inamdar, N.N. Chitosan-modifications and applications: Opportunities galore. React. Funct. Polym. 2008, 68, 1013–1051. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and Chitosan Preparation from Marine Sources. Structure, Properties and Applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mourya, V.K.; Inamdar, N.N. Trimethyl chitosan and its applications in drug delivery. J. Mater. Sci. Mater. Med. 2009, 20, 1057–1079. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Nasrollahzadeh, M.; Selva, M.; Issaabadi, Z.; Luque, R. Waste-to-wealth: Biowaste valorization into valuable bio(nano)materials. Chem. Soc. Rev. 2019, 48, 4791–4822. [Google Scholar] [CrossRef]
- Pillai, C.K.S.; Paul, W.; Sharma, C.P. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Ravi Kumar, M.N.V. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Qin, C.; Li, H.; Xiao, Q.; Liu, Y.; Zhu, J.; Du, Y. Water-solubility of chitosan and its antimicrobial activity. Carbohydr. Polym. 2006, 63, 367–374. [Google Scholar] [CrossRef]
- Sashiwa, H.; Aiba, S.-I. Chemically modified chitin and chitosan as biomaterials. Prog. Polym. Sci. 2004, 29, 887–908. [Google Scholar] [CrossRef]
- Carvalho, L.C.R.; Queda, F.; Santos, C.V.A.; Marques, M.M.B. Selective Modification of Chitin and Chitosan: En Route to Tailored Oligosaccharides. Chem. Asian J. 2016, 11, 3468–3481. [Google Scholar] [CrossRef]
- Wu, M.; Long, Z.; Xiao, H.; Dong, C. Recent research progress on preparation and application of N,N,N-trimethyl chitosan. Carbohydr. Res. 2016, 434, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Benediktsdóttir, B.E.; Gaware, V.S.; Rúnarsson, Ö.V.; Jónsdóttir, S.; Jensen, K.J.; Másson, M. Synthesis of N,N,N-trimethyl chitosan homopolymer and highly substituted N-alkyl-N, N-dimethyl chitosan derivatives with the aid of di-tert-butyldimethylsilyl chitosan. Carbohydr. Polym. 2011, 86, 1451–1460. [Google Scholar] [CrossRef]
- Rúnarsson, Ö.V.; Holappa, J.; Nevalainen, T.; Hjálmarsdóttir, M.; Järvinen, T.; Loftsson, T.; Einarsson, J.M.; Jónsdóttir, S.; Valdimarsdóttir, M.; Másson, M. Antibacterial activity of methylated chitosan and chitooligomer derivatives: Synthesis and structure activity relationships. Eur. Polym. J. 2007, 43, 2660–2671. [Google Scholar] [CrossRef]
- Jintapattanakit, A.; Mao, S.; Kissel, T.; Junyaprasert, V.B. Physicochemical properties and biocompatibility of N-trimethyl chitosan: Effect of quaternization and dimethylation. Eur. J. Pharm. Biopharm. 2008, 70, 563–571. [Google Scholar] [CrossRef]
- Kulkarni, A.D.; Patel, H.M.; Surana, S.J.; Vanjari, Y.H.; Belgamwar, V.S.; Pardeshi, C.V. N,N,N-Trimethyl chitosan: An advanced polymer with myriad of opportunities in nanomedicine. Carbohydr. Polym. 2017, 157, 875–902. [Google Scholar] [CrossRef]
- Rúnarsson, Ö.V.; Holappa, J.; Jónsdóttir, S.; Steinsson, H.; Másson, M. N-selective ‘one pot’ synthesis of highly N-substituted trimethyl chitosan (TMC). Carbohydr. Polym. 2008, 74, 740–744. [Google Scholar] [CrossRef]
- Sieval, A.B.; Thanou, M.; Kotze’, A.F.; Verhoef, J.C.; Brussee, J.; Junginger, H.E. Preparation and NMR characterization of highly substitutedN-trimethyl chitosan chloride. Carbohydr. Polym. 1998, 36, 157–165. [Google Scholar] [CrossRef]
- De Britto, D.; de Assis, O.B.G. Synthesis and mechanical properties of quaternary salts of chitosan-based films for food application. Int. J. Biol. Macromol. 2007, 41, 198–203. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A.; Tanfani, F. The N-permethylation of chitosan and the preparation of N-trimethyl chitosan iodide. Carbohydr. Polym. 1985, 5, 297–307. [Google Scholar] [CrossRef]
- Verheul, R.J.; Amidi, M.; van der Wal, S.; van Riet, E.; Jiskoot, W.; Hennink, W.E. Synthesis, characterization and in vitro biological properties of O-methyl free N,N,N-trimethylated chitosan. Biomaterials 2008, 29, 3642–3649. [Google Scholar] [CrossRef]
- Ono, Y. Catalysis in the production and reactions of dimethyl carbonate, an environmentally benign building block. Appl. Catal. A 1997, 155, 133–166. [Google Scholar] [CrossRef]
- Restani, P.; Galli, C.L. Oral Toxicity of Formaldehyde and Its Derivatives. Crit. Rev. Toxicol. 1991, 21, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Tundo, P.; Selva, M. The Chemistry of Dimethyl Carbonate. Acc. Chem. Res. 2002, 35, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Selva, M.; Perosa, A. Green chemistry metrics: A comparative evaluation of dimethyl carbonate, methyl iodide, dimethyl sulfate and methanol as methylating agents. Green Chem. 2008, 10, 457–464. [Google Scholar] [CrossRef]
- Bangde, P.S.; Jain, R.; Dandekar, P. Alternative Approach to Synthesize Methylated Chitosan Using Deep Eutectic Solvents, Biocatalyst and “Green” Methylating Agents. ACS Sustain. Chem. Eng. 2016, 4, 3552–3557. [Google Scholar] [CrossRef]
- Wu, M.; Long, Z.; Xiao, H.; Dong, C. Preparation of N,N,N-trimethyl chitosan via a novel approach using dimethyl carbonate. Carbohydr. Polym. 2017, 169, 83–91. [Google Scholar] [CrossRef]
- Olivier-Bourbigou, H.; Magna, L.; Morvan, D. Ionic liquids and catalysis: Recent progress from knowledge to applications. Appl. Catal. A 2010, 373, 1–56. [Google Scholar] [CrossRef]
- Olivier-Bourbigou, H.; Magna, L. Ionic liquids: Perspectives for organic and catalytic reactions. J. Mol. Catal. A Chem. 2002, 182–183, 419–437. [Google Scholar] [CrossRef]
- Gordon, C.M. New developments in catalysis using ionic liquids. Appl. Catal. A 2001, 222, 101–117. [Google Scholar] [CrossRef]
- Welton, T. Room-temperature ionic liquids. Solvents for synthesis and catalysis. Chem. Rev. 1999, 99, 2071–2083. [Google Scholar] [CrossRef]
- Hallett, J.P.; Welton, T. Room-Temperature Ionic Liquids: Solvents for Synthesis and Catalysis. 2. Chem. Rev. 2011, 111, 3508–3576. [Google Scholar] [CrossRef] [PubMed]
- Yoo, C.G.; Pu, Y.; Ragauskas, A.J. Ionic liquids: Promising green solvents for lignocellulosic biomass utilization. Curr. Opin. Green Sustain. Chem. 2017, 5, 5–11. [Google Scholar] [CrossRef]
- Fiorani, G.; Perosa, A.; Selva, M. Dimethyl carbonate: A versatile reagent for a sustainable valorization of renewables. Green Chem. 2018, 20, 288–322. [Google Scholar] [CrossRef]
- Zhang, P.B.; Fan, M.M.; Wei, X.Q.; Zeng, Y.N. A novel quaternary ammonium salt synthesis with dimethyl carbonate as alkylating agent. Optoelectron. Adv. Mater. Rapid Commun. 2011, 5, 164–166. [Google Scholar]
- Zheng, Z.; Wu, T.; Zheng, R.; Wu, Y.; Zhou, X. Study on the synthesis of quaternary ammonium salts using imidazolium ionic liquid as catalyst. Catal. Commun. 2007, 8, 39–42. [Google Scholar] [CrossRef]
- Zheng, Z.; Wu, T.; Zhou, X. The synthesis of quaternary ammonium salts from ammonium salts and dialkyl carbonate. Chem. Commun. 2006, 1864–1865. [Google Scholar] [CrossRef]
- Lavertu, M.; Xia, Z.; Serreqi, A.N.; Berrada, M.; Rodrigues, A.; Wang, D.; Buschmann, M.D.; Gupta, A. A validated 1H NMR method for the determination of the degree of deacetylation of chitosan. J. Pharm. Biomed. Anal. 2003, 32, 1149–1158. [Google Scholar] [CrossRef]
- Patrulea, V.; Applegate, L.A.; Ostafe, V.; Jordan, O.; Borchard, G. Optimized synthesis of O-carboxymethyl-N,N,N-trimethyl chitosan. Carbohydr. Polym. 2015, 122, 46–52. [Google Scholar] [CrossRef]
- Lim, L.-Y.; Khor, E.; Ling, C.-E. Effects of dry heat and saturated steam on the physical properties of chitosan. J. Biomed. Mater. Res. 1999, 48, 111–116. [Google Scholar] [CrossRef]
- Holme, H.K.; Foros, H.; Pettersen, H.; Dornish, M.; Smidsrød, O. Thermal depolymerization of chitosan chloride. Carbohydr. Polym. 2001, 46, 287–294. [Google Scholar] [CrossRef]
- Szymanska, E.; Winnicka, K. Stability of Chitosan-A Challenge for Pharmaceutical and Biomedical Applications. Mar. Drugs 2015, 13, 1819–1846. [Google Scholar] [CrossRef] [PubMed]
- Mucha, M.; Pawlak, A. Complex study on chitosan degradability. Polimery 2002, 47, 509–516. [Google Scholar] [CrossRef]
- Xie, J.; Wu, C.; Christopher, B.W.; Quan, J.; Zhu, L. Ionic Liquids—Promoted S-Methylation of Thiols Utilizing Dimethyl Carbonate AU—Xie, Jiangang. Phosphorus Sulfur Silicon Relat. Elem. 2011, 186, 31–37. [Google Scholar] [CrossRef]
- Zawadzki, J.; Kaczmarek, H. Thermal treatment of chitosan in various conditions. Carbohydr. Polym. 2010, 80, 394–400. [Google Scholar] [CrossRef]
- Senra, T.D.; Santos, D.M.; Desbrières, J.; Campana-Filho, S.P. Extensive N-methylation of chitosan: Evaluating the effects of the reaction conditions by using response surface methodology. Polym. Int. 2015, 64, 1617–1626. [Google Scholar] [CrossRef]
- Li, M.G.; Zang, H.J.; Feng, J.X.; Yan, Q.; Yu, N.Q.; Shi, X.L.; Cheng, B.W. Efficient conversion of chitosan into 5-hydroxymethylfurfural via hydrothermal synthesis in ionic liquids aqueous solution. Polym. Degrad. Stab. 2015, 121, 331–339. [Google Scholar] [CrossRef]
- Laus, G.; Bentivoglio, G.; Schottenberger, H.; Kahlenberg, V.; Kopacka, H.; Röder, T.; Sixta, H. Ionic Liquids: Current Developments, Potential and Drawbacks for Industrial Applications. Lenzing. Ber. 2005, 84, 71–85. [Google Scholar]
- Kärkkäinen, J.; Lappalainen, K.; Joensuu, P.; Lajunen, M. HPLC-ELSD analysis of six starch species heat-dispersed in [BMIM]Cl ionic liquid. Carbohydr. Polym. 2011, 84, 509–516. [Google Scholar] [CrossRef]
- De Britto, D.; Campana-Filho, S.P. A kinetic study on the thermal degradation of N,N,N-trimethylchitosan. Polym. Degrad. Stab. 2004, 84, 353–361. [Google Scholar] [CrossRef]
- Hamman, J.H.; Kotzé, A.F. Effect of the Type of Base and Number of Reaction Steps on the Degree of Quaternization and Molecular Weight of N-Trimethyl Chitosan Chloride. Drug Dev. Ind. Pharm. 2001, 27, 373–380. [Google Scholar] [CrossRef]




 ), dimethylation (DD,
), dimethylation (DD,  ) and quaternisation (DQ,
) and quaternisation (DQ,  ), and total degree of substitution (
), and total degree of substitution (  ) for TMC prepared using DMC as the methylating agent with varying reaction times (0–12 h).
) for TMC prepared using DMC as the methylating agent with varying reaction times (0–12 h).
 ), dimethylation (DD,
), dimethylation (DD,  ) and quaternisation (DQ,
) and quaternisation (DQ,  ), and total degree of substitution (
), and total degree of substitution (  ) for TMC prepared using DMC as the methylating agent with varying reaction times (0–12 h).
) for TMC prepared using DMC as the methylating agent with varying reaction times (0–12 h).
| Methylating Agent | DM (%) | DD (%) | DQ (%) | DAc (%) | Total (%) * |
|---|---|---|---|---|---|
| Methyl Iodide | 6.9 | 47.3 | 20.6 | 15.5 | 90.3 |
| DMC | 10.0 | 34.2 | 6.7 | 16.7 | 67.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hemming, E.B.; Masters, A.F.; Perosa, A.; Selva, M.; Maschmeyer, T. Single-Step Methylation of Chitosan Using Dimethyl Carbonate as a Green Methylating Agent. Molecules 2019, 24, 3986. https://doi.org/10.3390/molecules24213986
Hemming EB, Masters AF, Perosa A, Selva M, Maschmeyer T. Single-Step Methylation of Chitosan Using Dimethyl Carbonate as a Green Methylating Agent. Molecules. 2019; 24(21):3986. https://doi.org/10.3390/molecules24213986
Chicago/Turabian StyleHemming, Ellen B., Anthony F. Masters, Alvise Perosa, Maurizio Selva, and Thomas Maschmeyer. 2019. "Single-Step Methylation of Chitosan Using Dimethyl Carbonate as a Green Methylating Agent" Molecules 24, no. 21: 3986. https://doi.org/10.3390/molecules24213986
APA StyleHemming, E. B., Masters, A. F., Perosa, A., Selva, M., & Maschmeyer, T. (2019). Single-Step Methylation of Chitosan Using Dimethyl Carbonate as a Green Methylating Agent. Molecules, 24(21), 3986. https://doi.org/10.3390/molecules24213986