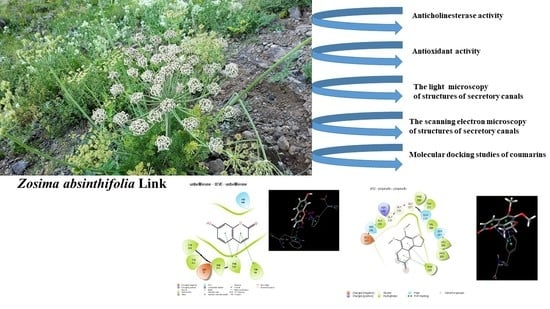
Molecular Docking Studies of Coumarins Isolated from Extracts and Essential Oils of Zosima absinthifolia Link as Potential Inhibitors for Alzheimer’s Disease
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Plant Specimens
4.2. Extraction and Isolation
4.3. Isolation of the Essential Oil, GC-FID and GC-MS Analyses
4.4. Determination of Total Phenolics
4.5. 1,1-Diphenyl-2-picrylhydrazyl (DPPH) Radical Scavenging Capacity Assay
4.6. Anti-Lipid Peroxidation Activity
4.7. Determination of AChE and BuChE Inhibition Activities
4.8. Microscopic Analysis
4.9. Molecular Docking Studies
4.10. Protein Preparation
4.11. Ligand Preparation
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Warren, B.Z.; Ira, T.L. Alzheimer’s Disease in Down Syndrome: Neurobıology And Risk. Ment. Retard. Dev. Disabil. Res. Rev. 2007, 13, 237–246. [Google Scholar]
- Karch, S.; Broichhagen, J.; Schneider, J.; Böning, D.; Hartmann, S.; Schmid, B.; Tripal, P.; Palmisano, R.; Alzheimer, C.; Johnsson, K.; Huth, T. A New Fluorogenic Small-Molecule Labeling Tool for Surface Diffusion Analysis and Advanced Fluorescence Imaging of β-Site Amyloid Precursor Protein-Cleaving Enzyme 1 Based on Silicone Rhodamine: SiR-BACE1. J. Med. Chem. 2018, 61, 6121–6139. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. The Epidemiology and Impact of Dementia; WHO: Geneva, Switzerland, 2015; Available online: http://www.who.int/mental_health/neurology/dementia/dementia_thematicbrief_epidemiology.pdf (accessed on 23 October 2018).
- Sadaoui, N.; Bec, N.; Barragan-Montero, V.; Kadrie, N.; Cuisinier, F.; Larroque, C.; Arab, K.; Khettal, B. The essential oil of Algerian Ammodaucus leucotrichus Coss. & Dur. and its effect on the cholinesterase and monoamine oxidase activities. Fitoterapia 2018, 130, 1–5. [Google Scholar] [PubMed]
- Ustun, O.; Senol, F.S.; Kurkcuoglu, M.; Orhan, I.E.; Kartal, M.; Baser, K.H.C. Investigation on chemical composition, anticholinesterase and antioxidant activities of extracts and essential oils of Turkish Pinus species and pycnogenol. Ind. Crops Prod. 2012, 38, 115–123. [Google Scholar] [CrossRef]
- Kim, J.; Lee, H.J.; Lee, K.W. Naturally occurring phytochemicals for the prevention of Alzheimer’s disease. J. Neurochem. 2010, 112, 1415–1430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, A.; Proenc, C.; Serralheiro, M.L.M.; Araujo, M.E.M. The in vitro screening for acetylcholinesterase inhibition and antioxidant activity of medicinal plants from Portugal. J. Ethnopharmacol. 2006, 108, 3–37. [Google Scholar] [CrossRef]
- Albanese, L.; Ciriminna, R.; Meneguzzo, F.; Pagliaro, M. Innovative beer-brewing of typical, old and healthy wheat varieties toboost their spreading. J. Clean. Prod. 2018, 171, 297–311. [Google Scholar] [CrossRef]
- Luz, D.A.; Pinheiro, A.M.; Silva, M.L.; Monteiroa, M.C.; Predige, R.D.; Maiaa, C.S.F.; Andrad, E.; Júniora, F. Ethnobotany, phytochemistry and neuropharmacological effects of Petiveria alliacea L. (Phytolaccaceae): A review. J. Ethnopharmacol. 2016, 185, 182–201. [Google Scholar] [CrossRef]
- Perry, E.K.; Pickering, A.T.; Wang, W.W.; Houghton, P.; Perry, N.L. Medicinal Plants and Alzheimer’s Disease: Integrating Ethnobotanical and Contemporary Scientific Evidence. J. Altern. Complement. Med. 1998, 4, 419–428. [Google Scholar] [CrossRef]
- Adsersen, A.; Gauguin, B.; Gudiksen, L.; Jäger, A.K. Screening of plants used in Danish folk medicine to treat memory dysfunction for acetylcholinesterase inhibitory activity. J. Ethnopharmacol. 2006, 104, 418–422. [Google Scholar] [CrossRef]
- Huang, W.Y.; Cai, Y.Z.; Zhang, Y. Natural Phenolic Compounds from Medicinal Herbs and Dietary Plants: Potential Use for Cancer Prevention. Nutr. Cancer 2009, 62, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Singh, B.; Singh, N. A review on coumarins as acetylcholinesterase inhibitors for Alzheimer’s disease. Bioorg. Med. Chem. 2012, 20, 1175–1180. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.T.H.; Guimaraes, I.M.; Flavia, R.S.; Fabiola, M.R. Alzheimer’s Disease: Targeting the Cholinergic System. Curr. Neuropharmacol. 2016, 14, 101–115. [Google Scholar] [CrossRef]
- Ciriminna, R.; Meneguzzo, F.; Fidalgo, A.; Ilharco, L.M.; Pagliaro, M. Extraction, benefits and valorization of olive polyphenols. Eur. J. Lipid Sci. Technol. 2016, 118, 503–511. [Google Scholar] [CrossRef]
- Mimica, N.D.; Bozin, B.; Sokovıc, M.; Simin, N. Antimicrobial and antioxidant activities of Melissa officinalis L. (Lamiaceae) Essential Oil. J. Agric. Food Chem. 2004, 52, 2485–2489. [Google Scholar] [CrossRef] [PubMed]
- Bahadir, O.; Citoglu, G.S.; Ozbek, H.; Dall’Acqua, S.; Hosek, J.; Smejkal, K. Hepatoprotective and TNF-alpha inhibitory activity of Zosima absinthifolia extracts and coumarins. Fitoterapia 2011, 82, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Bahadir, Ö.; Saltan, Ç.G.; Özbek, H. Evaluation of anti-inflammatory effect of Zosima absinthifolia and deltoin. J. Med. Plant. Res. 2010, 4, 909–914. [Google Scholar]
- Razavi, S.M.; Nejad-Ebrahimi, S. Chemical composition, allelopatic and antimicrobial potentials of the essential oil of Zosima absinthifolia (Vent.) Link fruits from Iran. Nat. Prod. Res. 2010, 24, 1125–1130. [Google Scholar] [CrossRef]
- Razavi, S.M.; Ghasemiyan, A.; Salehi, S.; Zahri, F. Screening of biological activity of Zosima absinthifolia fruits extracts. EurAsia J. BioSci. 2009, 4, 25–28. [Google Scholar] [CrossRef]
- Al-Shamma, A.; Mitscher, L.A. Comprehensive survey of indigenous Iraqi plants for potential economic value. 1. Screening results of 327 species for alkaloids and antimicrobial agents. J. Nat. Prod. 1979, 42, 633–642. [Google Scholar] [CrossRef]
- Reed, M.W.; Moore, H.W. Efficient Synthesis of Furochromone and Furocoumarin Natural Products (Khellin, Pimpinellin, Isophellopterin) by Thermal Rearrangement of 4-Furyl-4-hydroxycyclobutenones. J. Org. Chem. 1988, 53, 4166–4171. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andresjr, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef] [Green Version]
- Yerdelen, K.Ö.; Tosun, E. Synthesis, docking and biological evaluation of oxamide and fumaramide analogs as potential AChE and BuChE inhibitors. Med. Chem. Res. 2015, 24, 588–602. [Google Scholar] [CrossRef]
- Ali, Y.; Seong, H.; Reddy, M.R.; Seo, S.Y.; Choi, J.S.; Jung, H.A. Kinetics and Molecular Docking Studies of 6-Formyl Umbelliferone Isolated from Angelica decursiva as an Inhibitor of Cholinesterase and BACE1 Md. Molecules 2017, 22, 1604. [Google Scholar] [CrossRef] [PubMed]
- Karakaya, S.; Gözcü, S.; Güvenalp, Z.; Özbek, H.; Yuca, H.; Dursunoğlu, B.; Kazaz, C.; Kılıç, C.S. The α-amylase and α-glucosidase inhibitory activities of the dichloromethane extracts and constituents of Ferulago bracteata roots. Pharm. Biol. 2018, 56, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Razavi, S.; Imanzadeh, G.; Jahed, F.S.; Zarrini, G. Pyranocoumarins from Zosima absinthifolia (Vent) Link roots. Bioorg. Khim. 2013, 39, 244–246. [Google Scholar] [CrossRef] [PubMed]
- Sytar, O.; Bruckova, K.; Hunkova, E.; Zivcak, M.; Kiessoun, K.; Brestic, M. The application of Muliplex flourimetric sensor for analysis flavonoids content in the medical herbs family Asteraceae, Lamiaceae, Rosaceae. Biol. Res. 2015, 48, 48. [Google Scholar] [CrossRef]
- Granato, D.; Shahidi, F.; Wrolstad, R.; Kilmartin, P.; Melton, L.D.; Hidalgo, F.J.; Miyashita, K.; Camp, J.; Alasalvar, C.; Ismail, A.B.; et al. Antioxidant activity, total phenolics and flavonoids contents: Should we ban in vitro screening methods? Food Chem. 2018, 264, 471–475. [Google Scholar] [CrossRef]
- Đorđević, M.R.; Radulović, N.S.; Blagojević, P.D.; Pešić, M.S.; Akhlaghi, H. The essential oil of Zosima absinthifolia Link (Apiaceae) from Iran: A rich source of lavandulyl esters. Nat. Volatiles Essentıal Oils 2017, 4, 99. [Google Scholar]
- Kılıç, Ö. Essential Oil Composition of Two Apiaceae Species from Bingol (Turkey). Tr. J. Nat. Sci. 2014, 3, 18–21. [Google Scholar]
- Baser, K.H.C.; Ozek, T.; Demirci, B.; Kurkcuoglu, M.; Aytac, Z.; Duman, H. Composition of the essential oils of Zosima absinthifolia (Vent.) Link and Ferula elaeochytris Korovin from Turkey. Flavour Frag J. 2000, 15, 371–372. [Google Scholar] [CrossRef]
- Karthikeyan, R.; Kanimozhi, G.; Prasad, N.R.; Agilan, B.; Ganesan, M.; Srithar, G. Alpha pinene modulates UVA-induced oxidative stress, DNA damage and apoptosis in human skin epidermal keratinocytes. Life Sci. 2018, 212, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Villa, N.R.; Pacheco-Hernández, Y.; Becerra-Martínez, E.; Zárate-Reyes, J.A.; Cruz-Duráne, R. Chemical profile and pharmacological effects of the resin and essential oil from Bursera slechtendalii: A medicinal “copal tree” of southern Mexico. Fitoterapia 2018, 128, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Weston, R.J. Composition of Essential Oils from the Leaves of Seven New Zealand Species of Pittosporum (Pittosporaceae). J. Essential Oil Rese. 2004, 16, 453–458. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, C.; Ge, Z.; Liu, Y.; Liu, Y.; Feng, W.; Wei, T. Composition and potential anticancer activities of essential oils obtained from myrrh and frankincense. Oncol. Lett. 2013, 6, 1140–1146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheniclet, C.; Carde, J.P. Presence of Leucoplasts in Secretory Cells and of Monoterpenes in the Essential Oil: A Correlatıve Study. Isr. J. Bot. 1985, 34, 219–238. [Google Scholar]
- Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Scheffer, J.J.C. Factors affecting secondary metabolite production in plants: Volatile components and essential oils Factors affecting volatile and essential oil production in plants. Flavour Fragr. J. 2008, 23, 213–226. [Google Scholar] [CrossRef]
- Dickson, D.W. Neuropathological diagnosis of Alzheimer’s disease: A perspective from longitudinal clinicopathological studies. Neurobiol. Aging 1997, 18, 21–26. [Google Scholar] [CrossRef]
- Burcul, F.; Blazevic, I.; Radan, M.; Politeo, O. Terpenes, phenylpropanoids, sulfur and other essential oil constituents as inhibitors of cholinesterases. Curr. Med. Chem. 2018. [Google Scholar] [CrossRef]
- Karakaya, S.; Koca, M.; Kilic, C.S.; Coskun, M. Antioxidant and anticholinesterase activities of Ferulago syriaca Boiss. and F. isaurica Peșmen growing in Turkey. Med. Chem. Res. 2018, 27, 1843–1850. [Google Scholar] [CrossRef]
- Karakaya, S.; Göger, G.; Kılıç, C.S.; Demirci, B. Composition of volatile oil of the aerial parts, flowers and roots of Ferulago blancheana Post. (Apiaceae) growing in Turkey and determination of their antimicrobial activities by bioautography method. Turk. J. Pharm. Sci. 2016, 13, 173–180. [Google Scholar]
- Molyneux, P. The use of the stable free radical diphenylpicryl-hydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J. Sci. Technol. 2004, 26, 211–219. [Google Scholar]
- Sytar, O.; Bośko, P.; Živčák, M.; Brestic, M.; Smetanska, I. Bioactive Phytochemicals and Antioxidant Properties of the Grains and Sprouts of Colored Wheat Genotypes. Molecules 2018, 23, 2282. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimentry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Viticult. 1965, 16, 144–158. [Google Scholar]
- Dinis, T.C.P.; Madeira, V.M.C.; Almeida, L.M. Action of phenolic derivates (acetoaminophen, salycilate, and 5-aminosalycilate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch. Biochem. Biophys. 1994, 315, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Çelebioğlu, S.; Baytop, T. Bitkisel tozların tetkiki için yeni bir reaktif. Farmakolog 1949, 19, 301. [Google Scholar]
- ÖZKAN, Y. Türk Farmakopesi 2017; Genel monograflar I, T.C. Sağlık Bakanlığı Yayın No: 1098, 1.; Baskı: Ankara, Turkey, 2018. Available online: https://www.titck.gov.tr/Dosyalar/Laboratuvar/T%C3%BCrkFarmakopeDergisi2.Cilt1.Say%C4%B1s%C4%B1.pdf (accessed on 4 May 2016).
- Kryger, G.; Silman, I.; Sussman, J.L. Structure of acetylcholinesterase complexed with E2020 (Aricept): Implications for the design of new anti-Alzheimer drugs. Structure 1999, 7, 297–307. [Google Scholar] [CrossRef]
- Nicolet, Y.; Lockridge, O.; Masson, P.; Fontecilla-Camps, J.C.; Nachon, F. Crystal structure of human butyrylcholinesterase and of its complexes with substrate and products. J. Biol. Chem. 2003, 278, 41141–41147. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |









| Tested Samples | IC50 Values (µg/mL) ± SD * | |||
|---|---|---|---|---|
| Aerial Part | Root | Flower | Fruit | |
| MeOH | 204.16 ± 2.16 | 234.21 ± 4.26 | 199.43 ± 2.46 | 116.25 ± 3.51 |
| Hexane | 266.67 ± 2.97 | 198.15 ± 1.78 | 159.54 ± 1.48 | 500> |
| CH2Cl2 | 169.21 ± 4.22 | 500> | 301.53 ± 3.01 | 48.98 ± 2.45 |
| EtOAc | 255.21 ± 3.43 | 217.99 ± 4.35 | 478.90 ± 1.78 | 196.25 ± 2.66 |
| BuOH | 500> | 367.60 ± 3.21 | 298.33 ± 3.55 | 487.45 ± 3.12 |
| Aqueous residue | 500> | 500> | 500> | 500> |
| Essential oils | 225.17 ± 3.29 | 390.36 ± 1.56 | 97.11 ± 2.25 | 389.67 ± 2.86 |
| Bergapten | 56.99 ± 3.87 | |||
| Imperatorin | 79.23 ± 3.48 | |||
| Pimpinellin | 49.23 ± 2.19 | |||
| Umbelliferone | 79.53 ± 3.98 | |||
| Chlorogenic acid | 12.98 ± 4.89 | |||
| Propyl gallate | 3.44 ± 2.05 | |||
| Rutin | 9.65 ±3.09 | |||
| Samples | Enzymes | Percentile of inhibition ± S.E.M a against AChE and BuChE | |||
|---|---|---|---|---|---|
| Aerial Part | Root | Flower | Fruit | ||
| MeOH | AChE | 6.45 ± 2.33 | 9.58 ± 2.55 | c | b |
| BuChE | 14.44 ± 1.56 | 38.12 ± 4.05 | 27.33 ± 2.65 | 67.35 ± 1.56 | |
| Hexane | AChE | b | 3.25 ± 1.57 | c | c |
| BuChE | 17.35 ± 3.08 | 45.09 ± 2.66 | 24.97 ± 4.09 | 34.31 ± 2.76 | |
| CH2Cl2 | AChE | b | 29.15 ± 2.45 | c | 31.46 ± 2.78 |
| BuChE | 64.66 ± 2.56 | 71.32 ± 3.09 | 69.25 ± 4.10 | 82.27 ± 1.97 | |
| EtOAc | AChE | 3.34 ± 1.49 | 4.58 ± 4.66 | b | 9.03 ± 2.78 |
| BuChE | 29.09 ± 2.66 | 28.05 ± 2.13 | 36.21 ± 2.35 | 43.44 ± 3.15 | |
| BuOH | AChE | 3.55 ± 3.70 | c | 4.33 ± 1.65 | c |
| BuChE | 17.56 ± 2.54 | 14.54 ± 3.44 | 28.23 ± 2.54 | b | |
| Aqueous residue | AChE | c | b | c | b |
| BuChE | b | 9.42 ± 1.97 | b | 17.21 ± 2.45 | |
| Essential oils | AChE | b | 16.66 ± 3.21 | 6.45 ± 2.09 | 26.11 ± 2.13 |
| BuChE | 34.56 ± 2.47 | 56. 30 ± 3.51 | 78.65 ± 2.66 | 72.24 ± 2.44 | |
| Bergapten | AChE | 18.98 ± 2.98 | |||
| BuChE | 31.00 ± 3.02 | ||||
| Imperatorin | AChE | 20.44 ± 2.24 | |||
| BuChE | 44.23 ± 2.09 | ||||
| Pimpinellin | AChE | 23.54 ± 1.29 | |||
| BuChE | 66.55 ± 2.61 | ||||
| Umbelliferone | AChE | 61.09 ± 4.46 | |||
| BuChE | 40.99 ± 5.61 | ||||
| Donepezil | AChE | 82.45 ± 2.64 | |||
| BuChE | 90.33 ± 4.16 | ||||
| Used Parts | Crushed (g) | Yields | Colour | Collection Time |
|---|---|---|---|---|
| Aerial | 152 | 0.329 | Light yellow | 2017 |
| Root | 132 | 0.008 | White | 2017 |
| Flower | 35 | 0.057 | Yellow | 2018 |
| Fruit | 80 | 1.250 | Yellow | 2017 |
| RRI | Compound | Ap % | R % | Fl % | Fr % |
|---|---|---|---|---|---|
| 1032 | α-Pinene | 4.4 | 1.3 | 2.2 | 0.1 |
| 1048 | 2-Methyl-3-buten-2-ol | - | - | tr | tr |
| 1076 | Camphene | 0.2 | - | 0.1 | tr |
| 1093 | Hexanal | 0.3 | - | tr | - |
| 1118 | β-Pinene | 2.0 | 8.9 | 0.2 | 0.1 |
| 1132 | Sabinene | 0.3 | 0.1 | 0.1 | tr |
| 1151 | δ-4-Carene | - | - | 0.1 | - |
| 1174 | Myrcene | 1.0 | 3.0 | 1.3 | tr |
| 1176 | α-Phellandrene | 0.2 | 0.1 | - | - |
| 1194 | Heptanal | - | 0.4 | - | - |
| 1203 | Limonene | 1.8 | 2.7 | 1.5 | 0.1 |
| 1218 | β-Phellandrene | 1.0 | 0.4 | 0.7 | 0.1 |
| 1225 | (Z)-3-Hexenal | - | - | tr | - |
| 1244 | 2-Pentyl furan | - | 0.2 | 0.1 | tr |
| 1246 | (Z)-β-Ocimene | - | 0.3 | - | tr |
| 1255 | γ-Terpinene | - | 0.2 | tr | - |
| 1266 | (E)-β-Ocimene | - | - | 0.4 | - |
| 1280 | p-Cymene | 0.5 | 2.2 | 0.1 | - |
| 1290 | Terpinolene | 0.4 | 1.1 | 0.1 | tr |
| 1296 | Octanal | 0.3 | 2.5 | tr | 0.2 |
| 1348 | 6-Methyl-5-hepten-2-one | - | - | tr | - |
| 1360 | Hexanol | - | - | tr | - |
| 1398 | 2-Nonanone | - | 2.6 | - | |
| 1399 | Methyl octanoate | - | - | tr | - |
| 1400 | Nonanal | - | 0.3 | tr | - |
| 1444 | Ethyl octanoate | - | - | 0.2 | - |
| 1452 | α,p-Dimethylstyrene | - | 0.3 | - | - |
| 1483 | Octyl acetate | 7.3 | 1.0 | 19.9 | 81.6 |
| 1497 | α-Copaene | - | - | 0.1 | tr |
| 1506 | Decanal | - | - | - | 0.1 |
| 1516 | (Z)-4-Octenyl acetate | 0.3 | - | 0.5 | 5.1 |
| 1535 | β-Bourbonene | 1.3 | - | 0.1 | 0.3 |
| 1538 | trans-Chrysanthenyl acetate | - | - | 1.6 | - |
| 1553 | Linalool | - | - | 0.4 | 0.2 |
| 1562 | Octanol | 8.8 | 2.8 | 4.6 | 3.2 |
| 1571 | trans-p-Menth-2-en-1-ol | - | - | 1.5 | 0.1 |
| 1586 | Pinocarvone | - | 0.5 | - | - |
| 1589 | β-Ylangene | - | - | - | tr |
| 1591 | Bornyl acetate | 0.9 | 0.3 | 1.3 | 0.2 |
| 1597 | β-Copaene | - | - | - | 0.1 |
| 1600 | β-Elemene | - | - | - | tr |
| 1610 | Calarene (=β-gurjunene) | - | 0.2 | - | - |
| 1612 | β-Caryophyllene | 1.8 | 0.2 | 1.0 | 0.2 |
| 1614 | Carvacrol methyl ether (= methyl carvacrol) | - | 0.5 | - | - |
| 1623 | Octyl butyrate | 0.4 | - | 0.2 | 0.2 |
| 1634 | Octyl 2-methyl butyrate | 0.5 | - | 0.4 | 0.1 |
| 1638 | cis-p-Menth-2-en-1-ol | - | - | 0.7 | 0.1 |
| 1648 | Myrtenal | - | 0.4 | - | - |
| 1655 | (E)-2-Decenal | - | 1.5 | - | - |
| 1660 | (Z)-4-Octenyl butyrate | - | - | 0.2 | - |
| 1661 | trans-Pinocarvyl acetate | - | 26.7 | - | 0.1 |
| 1668 | Citronellyl acetate | - | - | 1.4 | 0.1 |
| 1670 | trans-Pinocarveol | - | 1.4 | - | - |
| 1687 | Decyl acetate | - | - | - | 0.1 |
| 1687 | α-Humulene | - | - | 0.1 | - |
| 1689 | trans-Piperitol | - | - | 0.4 | - |
| 1690 | Cryptone | - | - | 0.2 | - |
| 1704 | Myrtenyl acetate | - | 0.9 | - | - |
| 1706 | α-Terpineol | - | 0.3 | - | - |
| 1719 | Borneol | - | - | 0.1 | - |
| 1726 | Germacrene D | 2.3 | - | 2.0 | 0.5 |
| 1733 | Neryl acetate | - | - | 0.1 | - |
| 1747 | trans-Carvyl acetate | - | 0.2 | - | - |
| 1755 | Bicyclogermacrene | 0.7 | - | 0.7 | 0.1 |
| 1758 | cis-Piperitol | - | - | 0.5 | - |
| 1758 | (E,E)-α-Farnesene | - | - | 0.2 | - |
| 1772 | Citronellol | - | - | 0.4 | 0.1 |
| 1773 | δ-Cadinene | - | - | 0.1 | - |
| 1779 | (E,Z)-2,4-Decadienal | - | 0.3 | - | - |
| 1786 | ar-Curcumene | 0.2 | 0.3 | 0.2 | 0.1 |
| 1689 | trans-Piperitol | - | - | - | tr |
| 1804 | Myrtenol | - | 0.6 | - | - |
| 1827 | (E,E)-2,4-Decadienal | - | 0.9 | - | - |
| 1829 | Octyl hexanoate | 0.7 | - | 0.6 | 0.2 |
| 1849 | Cuparene | - | 0.6 | 0.1 | - |
| 1856 | (Z)-4-octenyl hexanoate | 0.7 | - | 0.7 | - |
| 1857 | Geraniol | - | - | 0.2 | 0.1 |
| 1868 | (E)-Geranyl acetone | - | 1.4 | 0.1 | - |
| 1878 | 2,5-Dimethoxy-p-cymene | - | 3.6 | tr | - |
| 1945 | 1,5-Epoxysalvial(4)14-ene | - | - | tr | - |
| 1958 | (E)-β-Ionone | - | - | 0.3 | - |
| 1981 | Heptanoic acid | - | 0.2 | - | - |
| 2000 | Citronellyl hexanoate | - | - | 0.3 | - |
| 2008 | Caryophyllene oxide | 1.9 | 0.8 | 0.4 | 0.1 |
| 2020 | Octyl octanoate | 7.6 | 0.8 | 0.3 | 0.9 |
| 2050 | (E)-Nerolidol | 0.8 | - | 0.1 | - |
| 2069 | Germacrene D-4β-ol | - | - | 0.3 | - |
| 2084 | Octanoic acid | - | - | - | 0.1 |
| 2100 | Heneicosane | - | - | 0.1 | - |
| 2127 | 10-epi-γ-Eudesmol | - | - | 0.1 | - |
| 2131 | Hexahydrofarnesyl acetone | 0.4 | - | 0.1 | tr |
| 2144 | Spathulenol | 2.7 | - | 0.5 | 0.1 |
| 2170 | β-Bisabolol | - | - | 0.1 | - |
| 2183 | γ-Decalactone | - | - | - | 0.1 |
| 2187 | T-Cadinol | - | - | 0.1 | - |
| 2192 | Nonanoic acid | - | - | - | 0.1 |
| 2200 | Docosane | - | - | 0.1 | - |
| 2209 | T-Muurolol | - | - | 0.2 | - |
| 2214 | (2E,6Z)-Farnesal | - | - | 0.1 | - |
| 2219 | δ-Cadinol (= torreyol) | - | - | 0.1 | - |
| 2247 | trans-α-Bergamotol | - | - | 0.1 | - |
| 2255 | α-Cadinol | - | - | 0.6 | - |
| 2271 | (2E,6E)-Farnesyl acetate | - | - | 2.3 | - |
| 2278 | (2E,6E)-Farnesal | - | - | 0.4 | - |
| 2300 | Tricosane | - | - | 0.7 | - |
| 2373 | Unknown I | 12.5 | 5.0 | 15.4 | 1.0 |
| 2369 | (2E,6E)-Farnesol | - | - | 1.7 | - |
| 2450 | Unknown II | 26.4 | 2.3 | 8.9 | 1.4 |
| 2500 | Pentacosane | - | - | - | 0.1 |
| 2503 | Dodecanoic acid | - | - | - | 0.2 |
| 2622 | Phytol | - | - | 0.5 | - |
| 2670 | Tetradecanoic acid | - | - | - | 1.0 |
| 2700 | Heptacosane | - | - | 0.5 | - |
| 2900 | Nonacosane | - | - | - | 0.2 |
| 2931 | Hexadecanoic acid | 4.1 | 1.3 | 0.5 | 0.4 |
| Total Identified | 55.8 | 74.3 | 58.2 | 96.8 | |
| Total | 94.7 | 81.6 | 82.5 | 99.2 |
| Compound Class | Ap % | R % | Fl % | Fr % |
|---|---|---|---|---|
| Esters | 9.4 | 29.1 | 27.9 | 87.5 |
| Alcohols | 8.8 | 5.1 | 8.8 | 3.8 |
| Aldehydes | 0.6 | 6.3 | 0.5 | 0.3 |
| Ketones | 0.4 | 4.5 | 0.7 | tr |
| Fatty acids+ esters | 13.1 | 2.3 | 2.6 | 2.9 |
| Terpene hydrocarbons | 18.1 | 21.9 | 11.4 | 1.7 |
| Oxygenated terpenes | 5.4 | 4.9 | 4.8 | 0.2 |
| Furans | - | 0.2 | 0.1 | tr |
| Alkanes | - | - | 1.4 | 0.3 |
| Lactones | - | - | - | 0.1 |
| Total Identified | 55.8 | 74.3 | 58.2 | 96.8 |
| Species | Extracts/Fractions | Aerial Part | Root | Flower | Fruit |
|---|---|---|---|---|---|
| Zosima absinthifolia | MeOH (g) | 25.01 | 29.88 | 23.92 | 85.98 |
| Hexane (g) | 3.28 | 4.05 | 2.98 | 11.88 | |
| CH2Cl2 (g) | 9.12 | 10.10 | 8.97 | 26.01 | |
| EtOAc (g) | 1.66 | 2.24 | 1.59 | 4.81 | |
| BuOH (g) | 4.92 | 5.86 | 4.77 | 18.57 | |
| Aqueous residue (g) | 5.02 | 3.22 | 4.98 | 6.96 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karakaya, S.; Koca, M.; Yılmaz, S.V.; Yıldırım, K.; Pınar, N.M.; Demirci, B.; Brestic, M.; Sytar, O. Molecular Docking Studies of Coumarins Isolated from Extracts and Essential Oils of Zosima absinthifolia Link as Potential Inhibitors for Alzheimer’s Disease. Molecules 2019, 24, 722. https://doi.org/10.3390/molecules24040722
Karakaya S, Koca M, Yılmaz SV, Yıldırım K, Pınar NM, Demirci B, Brestic M, Sytar O. Molecular Docking Studies of Coumarins Isolated from Extracts and Essential Oils of Zosima absinthifolia Link as Potential Inhibitors for Alzheimer’s Disease. Molecules. 2019; 24(4):722. https://doi.org/10.3390/molecules24040722
Chicago/Turabian StyleKarakaya, Songul, Mehmet Koca, Serdar Volkan Yılmaz, Kadir Yıldırım, Nur Münevver Pınar, Betül Demirci, Marian Brestic, and Oksana Sytar. 2019. "Molecular Docking Studies of Coumarins Isolated from Extracts and Essential Oils of Zosima absinthifolia Link as Potential Inhibitors for Alzheimer’s Disease" Molecules 24, no. 4: 722. https://doi.org/10.3390/molecules24040722
APA StyleKarakaya, S., Koca, M., Yılmaz, S. V., Yıldırım, K., Pınar, N. M., Demirci, B., Brestic, M., & Sytar, O. (2019). Molecular Docking Studies of Coumarins Isolated from Extracts and Essential Oils of Zosima absinthifolia Link as Potential Inhibitors for Alzheimer’s Disease. Molecules, 24(4), 722. https://doi.org/10.3390/molecules24040722