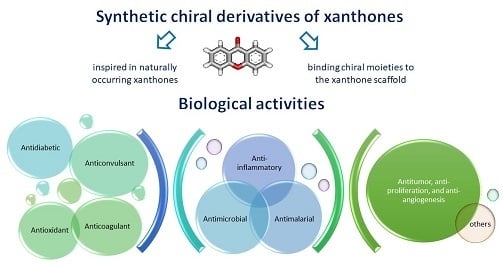
Synthetic Chiral Derivatives of Xanthones: Biological Activities and Enantioselectivity Studies
Abstract
:1. Introduction
2. Synthetic Chiral Derivatives of Xanthones
2.1. Synthetic CDXs Inspired in Naturally Occurring Xanthones
2.1.1. Synthetic Xanthonolignoids
2.1.2. Derivatives of Psorospermin
2.1.3. Derivatives of Muchimangins
2.1.4. Derivatives of Mangiferin
2.1.5. Derivatives of α-Mangostin
2.1.6. Derivatives of Caged Xanthones
2.2. Synthetic CDXs Obtained by Binding/Coupling Chiral Moieties to the Xanthone Scaffold
2.2.1. XAA and DMXAA Analogues
2.2.2. Synthetic Aminoalkanolic CDXs
2.2.3. CDXs Conjuged with Amines, Amino Esters and Amino Acids
2.2.4. CDXs Containing Piperazine Moieties and Analogues
2.2.5. CDXs Containing Other Moieties
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tiritan, M.E.; Ribeiro, A.R.; Fernandes, C.; Pinto, M. Chiral pharmaceuticals. In Kirk-Othmer Encyclopedia of Chemicl Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016. [Google Scholar]
- Calcaterra, A.; D’Acquarica, I. The market of chiral drugs: Chiral switches versus de novo enantiomerically pure compounds. J. Pharm. Biomed. Anal. 2018, 147, 323–340. [Google Scholar] [CrossRef] [PubMed]
- Caner, H.; Groner, E.; Levy, L.; Agranat, I. Trends in the development of chiral drugs. Drug Discov. Today 2004, 9, 105–110. [Google Scholar] [CrossRef]
- Blaser, H.U. Chirality and its implications for the pharmaceutical industry. Rend. Lincei. 2013, 24, 213–216. [Google Scholar] [CrossRef]
- Mannschreck, A.; Kiesswetter, R.; von Angerer, E. Unequal activities of enantiomers via biological receptors: Examples of chiral drug, pesticide, and fragrance molecules. J. Chem. Ed. 2007, 84, 2012–2017. [Google Scholar] [CrossRef]
- Triggle, D.J. Stereoselectivity of drug action. Drug Discov. Today 1997, 2, 138–147. [Google Scholar] [CrossRef]
- Cordato, D.J.; Mather, L.E.; Herkes, G.K. Stereochemistry in clinical medicine: A neurological perspective. J. Clin. Neurosci. 2003, 10, 649–654. [Google Scholar] [CrossRef]
- Smith, S.W. Chiral toxicology: It’s the same thing only different. Toxicol. Sci. 2009, 110, 4–30. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.; Fernandes, C.; Tiritan, M.E.; Pinto, M.M.M.; Valente, M.J.; Carvalho, M.; de Pinho, P.G.; Remião, F. Chiral enantioresolution of cathinone derivatives present in “legal highs”, and enantioselectivity evaluation on cytotoxicity of 3,4-methylenedioxypyrovalerone (mdpv). Forensic. Toxicol. 2016, 34, 372–385. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.; Fernandes, C.; de Pinho, P.G.; Remião, F. Chiral resolution and enantioselectivity of synthetic cathinones: A brief review. J. Anal. Toxicol. 2018, 42, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, J. Importance of stereospecific bioanalytical monitoring in drug development. J. Chromatogr. A 1996, 719, 3–13. [Google Scholar] [CrossRef]
- Andrushko, V.; Andrushko, N. Stereoselective Synthesis of Drugs and Natural Products; Jonh Wiley & Sons: Hoboken, NJ, USA, 2013; Volume 2, p. 1836. [Google Scholar]
- Bhadra, S.; Yamamoto, H. Substrate directed asymmetric reactions. Chem. Rev. 2018, 118, 3391–3446. [Google Scholar] [CrossRef] [PubMed]
- Karimi, B.; Tavakolian, M.; Akbari, M.; Mansouri, F. Ionic liquids in asymmetric synthesis: An overall view from reaction media to supported ionic liquid catalysis. ChemCatChem 2018, 10, 3173–3205. [Google Scholar] [CrossRef]
- Xue, Y.P.; Cao, C.H.; Zheng, Y.G. Enzymatic asymmetric synthesis of chiral amino acids. Chem. Soc. Rev. 2018, 47, 1516–1561. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.; Tiritan, M.E.; Pinto, M.M.M. Chiral separation in preparative scale: A brief overview of membranes as tools for enantiomeric separation. Symmetry 2017, 9, 206. [Google Scholar] [CrossRef]
- Ward, T.J.; Ward, K.D. Chiral separations: A review of current topics and trends. Anal. Chem. 2012, 84, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Francotte, E.R. Enantioselective chromatography as a powerful alternative for the preparation of drug enantiomers. J. Chromatogr. A 2001, 906, 379–397. [Google Scholar] [CrossRef]
- Haginaka, J. Pharmaceutical and biomedical applications of enantioseparations using liquid chromatographic techniques. J. Pharm. Biomed. Anal. 2002, 27, 357–372. [Google Scholar] [CrossRef]
- Council of the European Communities. Investigation of Chiral Active Substances. Directive 75/318/EEC; Council of the European Communities: Brussels, Belgium, 1993. [Google Scholar]
- FDA. Fda’s policy statement for the development of new stereoisomeric drugs. Fed. Reg. 1992, 57, 249. [Google Scholar]
- Shimazawa, R.; Nagai, N.; Toyoshima, S.; Okuda, H. Present state of new chiral drug development and review in Japan. J. Health Sci. 2008, 54, 23–29. [Google Scholar] [CrossRef]
- Fernandes, C.; Phyo, Y.Z.; Silva, A.S.; Tiritan, M.E.; Kijjoa, A.; Pinto, M.M.M. Chiral stationary phases based on small molecules: An update of the last 17 years. Sep. Purif. Rev. 2018, 47, 89–123. [Google Scholar] [CrossRef]
- Fernandes, C.; Tiritan, M.E.; Cravo, S.; Phyo, Y.Z.; Kijjoa, A.; Silva, A.M.S.; Cass, Q.B.; Pinto, M.M.M. New chiral stationary phases based on xanthone derivatives for liquid chromatography. Chirality 2017, 29, 430–442. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.; Tiritan, M.E.; Pinto, M.M.M.; Fernandes, C. Chiral stationary phases for liquid chromatography based on chitin- and chitosan-derived marine polysaccharides. Symmetry 2017, 9, 190. [Google Scholar] [CrossRef]
- Lämmerhofer, M. Chiral recognition by enantioselective liquid chromatography: Mechanisms and modern chiral stationary phases. J. Chromatogr. A 2010, 1217, 814–856. [Google Scholar] [CrossRef] [PubMed]
- Gales, L.; Damas, A.M. Xanthones–a structural perspective. Curr. Med. Chem. 2005, 12, 2499–2515. [Google Scholar] [CrossRef] [PubMed]
- Muthukrishnan, M.; Basavanag, U.M.V.; Puranik, V.G. The first ionic liquid-promoted kabbe condensation reaction for an expeditious synthesis of privileged bis-spirochromanone scaffolds. Tetrahedron. Lett. 2009, 50, 2643–2648. [Google Scholar] [CrossRef]
- Horton, D.A.; Bourne, G.T.; Smythe, M.L. The combinatorial synthesis of bicyclic privileged structures or privileged substructures. Chem. Rev. 2003, 103, 893–930. [Google Scholar] [CrossRef] [PubMed]
- Waseem, S.; Ahmad, I. Recent insight into the biological activities of synthetic xanthone derivatives. Eur. J. Med. Chem. 2016, 116, 267–280. [Google Scholar]
- Pinto, M.; Sousa, M.; Nascimento, M. Xanthone derivatives: New insights in biological activities. Curr. Med. Chem. 2005, 12, 2517–2538. [Google Scholar] [CrossRef]
- Wezeman, T.; Brase, S.; Masters, K.S. Xanthone dimers: A compound family which is both common and privileged. Nat. Prod. Rep. 2015, 32, 6–28. [Google Scholar] [CrossRef] [PubMed]
- Na, Y. Recent cancer drug development with xanthone structures. J. Pharm. Pharmacol. 2009, 61, 707–712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro, J.; Veloso, C.; Fernandes, C.; Tiritan, M.E.; Pinto, M.M.M. Carboxyxanthones: Bioactive agents and molecular scaffold for synthesis of analogues and derivatives. Molecules 2019, 24, 180. [Google Scholar] [CrossRef] [PubMed]
- Sathyadevi, P.; Chen, Y.J.; Wu, S.C.; Chen, Y.H.; Wang, Y.M. Reaction-based epoxide fluorescent probe for in vivo visualization of hydrogen sulfide. Biosens. Bioelectron. 2015, 68, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Takashima, I.; Kawagoe, R.; Hamachi, I.; Ojida, A. Development of an and logic-gate-type fluorescent probe for ratiometric imaging of autolysosome in cell autophagy. Chem. Eur. J. 2015, 21, 2038–2044. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.; Tiritan, M.E.; Pinto, M.M.M. Chiral derivatives of xanthones: Applications in medicinal chemistry and a new approach in liquid chromatography. Sci. Chromatogr. 2015, 7, 1–14. [Google Scholar]
- Masters, K.S.; Brase, S. Xanthones from fungi, lichens, and bacteria: The natural products and their synthesis. Chem. Rev. 2012, 112, 3717–3776. [Google Scholar] [CrossRef] [PubMed]
- Vieira, L.M.; Kijjoa, A. Naturally-occurring xanthones: Recent developments. Curr. Med. Chem. 2005, 12, 2413–2446. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.M.M.; Castanheiro, R.A.P.; Kijjoa, A. Xanthones from marine-derived microorganisms: Isolation, structure elucidation, and biological activities. In Encyclopedia of Analytical Chemistry, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA, 2014; Volume 27, pp. 1–21. [Google Scholar]
- Sousa, M.; Pinto, M. Synthesis of xanthones: An overview. Curr. Med. Chem. 2005, 12, 2447–2479. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, C.; Afonso, C.; Pinto, M. Routes to xanthones: An update on the synthetic approaches. Curr. Org. Chem. 2012, 16, 2818–2867. [Google Scholar] [CrossRef]
- Lanzotti, V. Drugs based on natural compounds: Recent achievements and future perspectives. Phytochem. Rev. 2014, 13, 725–726. [Google Scholar] [CrossRef]
- Michael, A. On the action of aromatic oxy-acids on phenols. Am. Chem. J. 1883, 5, 81–97. [Google Scholar]
- V Kostanecki, S. Über das gentisin. Monatsh. Chem. 1891, 12, 205–210. [Google Scholar] [CrossRef]
- Goldberg, I. Ueber Phenylirungen bei Gegenwart von Kupfer als Katalysator. Chem. Ber. 1906, 39, 1691–1692. [Google Scholar] [CrossRef]
- Chen, M.T.; Kuoh, Y.P.; Wang, C.H.; Chen, C.M.; Kuoh, C.S. Additional constituents of hypericum subalatum. J. Chin. Chem. Soc. 1988, 36, 165–168. [Google Scholar] [CrossRef]
- Wu, Q.-L.; Wang, S.-P.; Du, L.-J.; Yang, J.-S.; Xiao, P.-G. Xanthones from hypericum japonicum and h. Henryi. Phytochemistry 1998, 49, 1395–1402. [Google Scholar] [CrossRef]
- Pinto, M.; Sousa, E. Natural and synthetic xanthonolignoids: Chemistry and biological activities. Curr. Med. Chem. 2003, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, O.R.; Mesquita, A.A.L.; Silva, E.M.; Melo, T. Xanthones of kielmeyera ferruginea. Phytochemistry 1969, 8, 665–666. [Google Scholar] [CrossRef]
- Pinto, M.M.D.M.; Mesquita, A.A.L.; Gottlieb, O.R. Xanthonolignoids from kielmeyera coriacea. Phytochemistry 1987, 26, 2045–2048. [Google Scholar] [CrossRef]
- Tanaka, H.; Ishihara, M.; Ichino, K.; Ohiwa, N.; Ito, K. Total synthesis of a xanthonolignoid, kielcorin. Chem. Pharm. Bull. 1989, 37, 1916–1918. [Google Scholar] [CrossRef]
- Vishwakarma, R.; Kapil, R.S.; Popli, S.P. Total synthesis of Keilcorin. Indian J. Chem. Sect. B 1986, 25, 1021. [Google Scholar]
- Fernandes, E.G.R.; Pinto, M.M.M.; Silva, A.M.S.; Cavaleiro, J.A.S.; Gottlieb, O.R. Synthesis and structural elucidation of xanthonolignoids: Trans-(±)- kielcorin b and trans-(±)-isokielcorin b. Heterocycles 1999, 51, 821–828. [Google Scholar]
- Saraiva, L.; Fresco, P.; Pinto, E.; Sousa, E.; Pinto, M.; Gonçalves, J. Synthesis and in vivo modulatory activity of protein kinase c of xanthone derivatives. Bioorg. Med. Chem. 2002, 10, 3219–3227. [Google Scholar] [CrossRef]
- Sousa, E.P.; Silva, A.; Pinto, M.M.; Pedro, M.M.; Cerqueira, F.A.; Nascimento, M.S. Isomeric kielcorins and dihydroxyxanthones: Synthesis, structure elucidation, and inhibitory activities of growth of human cancer cell lines and on the proliferation of human lymphocytes in vitro. Helv. Chim. Acta 2002, 85, 2862–2876. [Google Scholar] [CrossRef]
- Saraiva, L.; Fresco, P.; Pinto, E.; Sousa, E.; Pinto, M.; Gonçalves, J. Inhibition of α, βi, δ, η and ζ protein kinase c isoforms by xanthonolignoids. J. Enzyme. Inhib. Med. Chem. 2003, 18, 357–370. [Google Scholar] [CrossRef] [PubMed]
- Fernandas, E.R.; Carvalho, F.D.; Remião, F.G.; Bastos, M.L.; Pinto, M.M.; Gottlieb, O.R. Hepatoprotective activity of xanthones and xanthonolignoids against tert-butylhydroperoxide-induced toxicity in isolated rat hepatocytes—comparison with silybin. Pharm. Res. 1995, 12, 1756–1760. [Google Scholar] [CrossRef]
- Sousa, E.P.; Tiritan, M.; Oliveira, R.V.; Afonso, C.; Cass, Q.; Pinto, M.M. Enantiomeric resolution of kielcorin derivatives by hplc on polysaccharide stationary phases using multimodal elution. Chirality 2004, 16, 279–285. [Google Scholar] [CrossRef]
- Sousa, M.E.; Tiritan, M.E.; Belaz, K.R.A.; Pedro, M.; Nascimento, M.S.J.; Cass, Q.B.; Pinto, M.M.M. Multimilligram enantioresolution of low-solubility xanthonolignoids on polysaccharide chiral stationary phases using a solid-phase injection system. J. Chromatogr. A 2006, 1120, 75–81. [Google Scholar] [CrossRef]
- Kupchan, S.M.; Streelman, D.R.; Sneden, A.T. Psorospermin, a new antileukemic xanthone from psorospermum febrifugum. J. Nat. Prod. 1980, 43, 296–301. [Google Scholar] [CrossRef]
- Schwaebe, M.K.; Moran, T.J.; Whitten, J.P. Total synthesis of psorospermin. Tetrahedron. Lett. 2005, 46, 827–829. [Google Scholar] [CrossRef]
- Heald, R.A.; Dexheimer, T.S.; Vankayalapati, H.; Siddiqui-Jain, A.; Szabo, L.Z.; Gleason-Guzman, M.C.; Hurley, L.H. Conformationally restricted analogues of psorospermin: Design, synthesis, and bioactivity of natural-product-related bisfuranoxanthones. J. Med. Chem. 2005, 48, 2993–3004. [Google Scholar] [CrossRef] [PubMed]
- Fellows, I.M.; Schwaebe, M.; Dexheimer, T.S.; Vankayalapati, H.; Gleason-Guzman, M.; Whitten, J.P.; Hurley, L.H. Determination of the importance of the stereochemistry of psorospermin in topoisomerase ii–induced alkylation of DNA and in vitro and in vivo biological activity. Mol. Cancer Ther. 2005, 4, 1729–1739. [Google Scholar] [CrossRef] [PubMed]
- Ayensu, E.S. Medicinal Plants of West Africa; Reference Publications Inc.: Algonac, MI, USA, 1978. [Google Scholar]
- Kodama, T.; Ito, T.; Dibwe, D.F.; Woo, S.Y.; Morita, H. Syntheses of benzophenone-xanthone hybrid polyketides and their antibacterial activities. Bioorg. Med. Chem. Lett. 2017, 27, 2397–2400. [Google Scholar] [CrossRef]
- Dibwe, D.; Awale, S.; Kadota, S.; Tezuka, Y. Muchimangins a–d: Novel diphenylmethyl-substituted xanthones from securidaca longepedunculata. Tetrahedron. Lett. 2012, 53, 6186–6190. [Google Scholar] [CrossRef]
- Gold-Smith, F.; Fernandez, A.; Bishop, K. Mangiferin and cancer: Mechanisms of action. Nutrients 2016, 8, 396. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Lu, Z. Antiviral effect of mangiferin and isomangiferin on herpes simplex virus. Chin. Med. J. 1990, 103, 160–165. [Google Scholar] [PubMed]
- Zhu, X.; Song, J.; Huang, Z.; Wu, Y.; Yu, M. Antiviral activity of mangiferin against herpes simplex virus type 2 in vitro. Acta Pol. Pharm. Sinica. 1993, 14, 452–454. [Google Scholar]
- Yoosook, C.; Bunyapraphatsara, N.; Boonyakiat, Y.; Kantasuk, C. Anti-herpes simplex virus activities of crude water extracts of thai medicinal plants. Phytomedicine 2000, 6, 411–419. [Google Scholar] [CrossRef]
- Benard, O.; Chi, Y. Medicinal properties of mangiferin, structural features, derivative synthesis, pharmacokinetics and biological activities. Mini-Rev. Med. Chem. 2015, 15, 582–594. [Google Scholar] [CrossRef]
- Imran, M.; Arshad, M.S.; Butt, M.S.; Kwon, J.H.; Arshad, M.U.; Sultan, M.T. Mangiferin: A natural miracle bioactive compound against lifestyle related disorders. Lip. Health Dis. 2017, 16, 84. [Google Scholar] [CrossRef]
- Araújo, J.; Fernandes, C.; Pinto, M.; Tiritan, M. Chiral derivatives of xanthones with antimicrobial activity. Molecules 2019, 24, 314. [Google Scholar] [CrossRef]
- Singh, S.; Tiwari, R.; Sinha, S.; Danta, C.; Prasad, S. Antimicrobial evaluation of mangiferin and its synthesized analogues. Asian Pac. J. Trop. Biomed. 2012, 2, S884–S887. [Google Scholar] [CrossRef]
- Singh, S.; Sinha, S.; Prasad, S.; Kumar, R.; Bithu, B.; Kumar, S.; Singh, P. Synthesis and evaluation of novel analogues of mangiferin as potent antipyretic. Asian Pac. J. Trop. Biomed. 2011, 4, 866–869. [Google Scholar] [CrossRef] [Green Version]
- Dar, A.; Faizi, S.; Naqvi, S.; Roome, T.; Zikr-ur-Rehman, S.; Ali, M.; Firdous, S.; Moin, S. Analgesic and antioxidant activity of mangiferin and its derivatives: The structure activity relationship. Biol. Pharm. Bull. 2005, 28, 596–600. [Google Scholar] [CrossRef] [PubMed]
- Mahendran, S.; Badami, S.; Ravi, S.; Thippeswamy, B.; Veerapur, V. Synthesis and evaluation of analgesic and anti-inflammatory activities of most active free radical scavenging derivatives of mangiferin. Bri. J. Appl. Sci. Technol. 2014, 4, 4959–4973. [Google Scholar] [CrossRef]
- Miura, T.; Ichiki, H.; Iwamoto, N.; Kato, M.; Kubo, M.; Sasaki, H.; Okada, M.; Ishida, T.; Seino, Y.; Tanigawa, K. Antidiabetic activity of the rhizoma of anemarrhena asphodeloides and active components, mangiferin and its glucoside. Biol. Pharm. Bull. 2001, 24, 1009–1011. [Google Scholar] [CrossRef] [PubMed]
- Miura, T.; Ichiki, H.; Hashimoto, I.; Iwamoto, N.; Kao, M.; Kubo, M.; Ishihara, E.; Komatsu, Y.; Okada, M.; Ishida, T.; et al. Antidiabetic activity of a xanthone compound, mangiferin. Phytomedicine 2001, 8, 85–87. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.G.; Wang, M.J.; Zhao, Q.J.; Yu, S.C.; Liu, C.M.; Wu, Q.Y. Synthesis of mangiferin derivates and study their potent ptp1b inhibitory activity. Chin. Chem. Lett. 2007, 18, 1323–1326. [Google Scholar] [CrossRef]
- Hu, H.; Wang, M.; Zhao, Q.; Liao, H.; Cai, L.; Song, Y.; Zhang, J.; Yu, S.; Chen, W.; Liu, C.; et al. Synthesis of mangiferin derivatives as protein tyrosine phosphatase 1b inhibitors. Chem. Nat. Compd. 2007, 43. [Google Scholar] [CrossRef]
- Li, X.; Du, Z.; Huang, Y.; Liu, B.; Hu, W.; Lu, W.; Deng, J. Synthesis and hypoglycemic activity of esterified-derivatives of mangiferin. Chin. J. Nat. Med. 2013, 11, 296–301. [Google Scholar] [CrossRef]
- Klaman, L.; Boss, O.; Peroni, O.; Kim, J.; Martino, J.; Zabolotny, J.; Moghal, N.; Lubkin, M.; Kim, Y.; Sharpe, A.; et al. Increased energy expenditure, decreased adiposity, and tissue-specific insulin sensitivity in protein-tyrosine phosphatase 1b-deficient mice. Mol. Cell. Biol. 2000, 20, 5479–5489. [Google Scholar] [CrossRef]
- Elchebly, M.; Payette, P.; Michaliszyn, E.; Cromlish, W.; Collins, S.; Loy, A.; Normandin, D.; Cheng, A.; Himms-Hagen, J.; Chan, C.; et al. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1b gene. Science 1999, 283, 1544–1548. [Google Scholar] [CrossRef]
- Correia-da-Silva, M.; Sousa, E.; Duarte, B.; Marques, F.; Carvalho, F.; Cunha-Ribeiro, L.; Pinto, M. Polysulfated xanthones: Multipathway development of a new generation of dual anticoagulant/antiplatelet agents. J. Med. Chem. 2011, 54, 5373–5384. [Google Scholar] [CrossRef] [PubMed]
- Kaomongkolgit, R.; Jamdez, K.; Chaisomboon, N. Antifungal activity of alpha-mangostin against candida albicans. J. Oral. Sci. 2009, 51, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Su, B.; Keller, W.; Mehta, R.; Kinghorn, A. Antioxidant xanthones from the pericarp of garcinia mangostana (mangosteen). J. Agric. Food. Chem. 2006, 54. [Google Scholar] [CrossRef] [PubMed]
- Guzman-Beltran, S.; Rubio-Badillo, M.A.; Juarez, E.; Hernandez-Sanchez, F.; Torres, M. Nordihydroguaiaretic acid (ndga) and alpha-mangostin inhibit the growth of mycobacterium tuberculosis by inducing autophagy. Int. Immunopharmacol. 2016, 31, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Huang, L.; Chen, X.H.; Zhu, X.F.; Qian, X.J.; Feng, G.K.; Lan, W.J.; Li, H.J. Cytotoxic prenylated xanthones from the pericarps of garcinia mangostana. Molecules 2014, 19, 1820–1827. [Google Scholar] [CrossRef]
- Sudta, P.; Jiarawapi, P.; Suksamrarn, A.; Hongmanee, P.; Suksamrarn, S. Potent activity against multidrug-resistant mycobacterium tuberculosis of α-mangostin analogs. Chem. Pharm. Bull. 2013, 61, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, L.; Wang, C. Anti-inflammatory activity of mangostins from garcinia mangostana. Food Chem. Toxicol. 2008, 46, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Iikubo, K.; Ishikawa, Y.; Ando, N.; Umezawab, K.; Nishiyama, S. The first direct synthesis of alfa-mangostin, a potent inhibitor of the acidic sphingomyelinase. Tetrahedron. Lett. 2002, 43. [Google Scholar] [CrossRef]
- Suksamrarn, S.; Suwannapoch, N.; Phakhodee, W.; Thanuhiranlert, J.; Ratananukul, P.; Chimnoi, N.; Suksamrarn, A. Antimycobacterial activity of prenylated xanthones from the fruits of garcinia mangostana. Chem. Pharm. Bull. 2003, 51, 857–859. [Google Scholar] [CrossRef] [PubMed]
- Koh, J.J.; Zou, H.; Mukherjee, D.; Lin, S.; Lim, F.; Tan, J.K.; Tan, D.; Stocker, B.L.; Timmer, M.; Corkran, H.M.; et al. Amphiphilic xanthones as a potent chemical entity of anti-mycobacterial agents with membrane-targeting properties. Eur. J. Med. Chem. 2016, 123, 684–703. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, S.; Koh, J.J.; Zou, H.; Lakshminarayanan, R.; Bai, Y.; Pervushin, K.; Zhou, L.; Verma, C.; Beuerman, R.W. A novel fragment based strategy for membrane active antimicrobials against mrsa. BBA-Biomembranes 2015, 1848, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Koh, J.J.; Aung, T.T.; Lim, F.; Li, J.; Zou, H.; Wang, L.; Lakshminarayanan, R.; Verma, C.; Wang, Y.; et al. Symmetrically substituted xanthone amphiphiles combat gram-positive bacterial resistance with enhanced membrane selectivity. J. Med. Chem. 2017, 60, 1362–1378. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Koh, J.J.; Li, J.; Qiu, S.; Aung, T.T.; Lin, H.; Lakshminarayanan, R.; Dai, X.; Tang, C.; Lim, F.H.; et al. Design and synthesis of amphiphilic xanthone-based, membrane-targeting antimicrobials with improved membrane selectivity. J. Med. Chem. 2013, 56, 2359–2373. [Google Scholar] [CrossRef] [PubMed]
- Koh, J.J.; Lin, S.; Aung, T.T.; Lim, F.; Zou, H.; Bai, Y.; Li, J.; Lin, H.; Pang, L.M.; Koh, W.L.; et al. Amino acid modified xanthone derivatives: Novel, highly promising membrane-active antimicrobials for multidrug-resistant gram-positive bacterial infections. J. Med. Chem. 2015, 58, 739–752. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Lantvit, D.D.; Carcache de Blanco, E.J.; Kardono, L.B.; Riswan, S.; Chai, H.; Cottrell, C.E.; Farnsworth, N.R.; Swanson, S.M.; Ding, Y.; et al. Proteasome-inhibitory and cytotoxic constituents of garcinia lateriflora: Absolute configuration of caged xanthones. Tetrahedron 2010, 66, 5311–5320. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Yuan, C.; Chai, H.B.; Ding, Y.; Li, X.C.; Ferreira, D.; Kinghorn, A.D. Absolute configuration of (−)-gambogic acid, an antitumor agent. J. Nat. Prod. 2011, 74, 460–463. [Google Scholar] [CrossRef] [PubMed]
- Rukachaisirikul, V.; Kaewnok, W.; Koysomboon, S.; Phongpaichit, S.; Taylor, W.C. Caged-tetraprenylated xanthones from garcinia scortechinii. Tetrahedron 2000, 56, 8539–8543. [Google Scholar] [CrossRef]
- Sukpondma, Y.; Rukachaisirikul, V.; Phongpaichit, S. Antibacterial caged-tetraprenylated xanthones from the fruits of garcinia hanburyi. Chem. Pharm. Bull. 2005, 53, 850–852. [Google Scholar] [CrossRef] [PubMed]
- Banik, K.; Harsha, C.; Bordoloi, D.; Lalduhsaki Sailo, B.; Sethi, G.; Leong, H.C.; Arfuso, F.; Mishra, S.; Wang, L.; Kumar, A.P.; et al. Therapeutic potential of gambogic acid, a caged xanthone, to target cancer. Cancer Lett. 2018, 416, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Chaiyakunvat, P.; Anantachoke, N.; Reutrakul, V.; Jiarpinitnun, C. Caged xanthones: Potent inhibitors of global predominant mrsa usa300. Bioorg. Med. Chem. Lett. 2016, 26, 2980–2983. [Google Scholar] [CrossRef]
- Ke, H.; Morrisey, J.M.; Qu, S.; Chantarasriwong, O.; Mather, M.W.; Theodorakis, E.A.; Vaidya, A.B. Caged garcinia xanthones, a novel chemical scaffold with potent antimalarial activity. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Reutrakul, V.; Anantachoke, N.; Pohmakotr, M.; Jaipetch, T.; Sophasan, S.; Yoosook, C.; Kasisit, J.; Napaswat, C.; Santisuk, T.; Tuchinda, P. Cytotoxic and anti-hiv-1 caged xanthones from the resin and fruits of garcinia hanburyi. Planta Med. 2007, 73, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.; Khan, F. Virtual screening, docking, admet and system pharmacology studies on garcinia caged xanthone derivatives for anticancer activity. Sci. Rep. 2018, 8, 5524. [Google Scholar] [CrossRef] [PubMed]
- Batova, A.; Altomare, D.; Chantarasriwong, O.; Ohlsen, K.L.; Creek, K.E.; Lin, Y.C.; Messersmith, A.; Yu, A.L.; Yu, J.; Theodorakis, E.A. The synthetic caged garcinia xanthone cluvenone induces cell stress and apoptosis and has immune modulatory activity. Mol. Cancer Ther. 2010, 9, 2869–2878. [Google Scholar] [CrossRef] [PubMed]
- Chantarasriwong, O.; Cho, W.C.; Batova, A.; Chavasiri, W.; Moore, C.; Rheingold, A.L.; Theodorakis, E.A. Evaluation of the pharmacophoric motif of the caged garcinia xanthones. Org. Biomol. Chem. 2009, 7, 4886–4894. [Google Scholar] [CrossRef]
- Batova, A.; Lam, T.; Wascholowski, V.; Yu, A.L.; Giannis, A.; Theodorakis, E.A. Synthesis and evaluation of caged garcinia xanthones. Org. Biomol. Chem. 2007, 5, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Elbel, K.M.; Guizzunti, G.; Theodoraki, M.A.; Xu, J.; Batova, A.; Dakanali, M.; Theodorakis, E.A. A-ring oxygenation modulates the chemistry and bioactivity of caged garcinia xanthones. Org. Biomol. Chem. 2013, 11, 3341–3348. [Google Scholar] [CrossRef]
- Li, X.; Wu, Y.; Wang, Y.; You, Q.; Zhang, X. ‘Click chemistry’ synthesis of novel natural product-like caged xanthones bearing a 1,2,3-triazole moiety with improved druglike properties as orally active antitumor agents. Molecules 2017, 22, 1834. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Wang, X.; Li, N.; Lin, C.; Gao, Y.; Yu, Z.; Guo, Q.; You, Q. Synthesis and anti-tumor evaluation of b-ring modified caged xanthone analogues of gambogic acid. Chin. J. Chem. 2012, 30, 35–42. [Google Scholar] [CrossRef]
- Wu, Y.; Hu, M.; Yang, L.; Li, X.; Bian, J.; Jiang, F.; Sun, H.; You, Q.; Zhang, X. Novel natural-product-like caged xanthones with improved druglike properties and in vivo antitumor potency. Bioorg. Med. Chem. Lett. 2015, 25, 2584–2588. [Google Scholar] [CrossRef]
- Xu, X.; Wu, Y.; Hu, M.; Li, X.; Bao, Q.; Bian, J.; You, Q.; Zhang, X. Novel natural product-like caged xanthones bearing a carbamate moiety exhibit antitumor potency and anti-angiogenesis activity in vivo. Sci. Rep. 2016, 6, 35771. [Google Scholar] [CrossRef] [PubMed]
- Miao, G.; Ma, J.; Yang, K.; Huang, Z.; Gu, Q.; Wang, Y.; Guo, Q.; You, Q.; Wang, J. Synthesis and bioevaluation of novel oxa-caged garcinia xanthones as anti-tumour agents. Aust. J. Chem. 2015, 68, 872. [Google Scholar] [CrossRef]
- Koh, J.J.; Lin, S.; Bai, Y.; Sin, W.; Aung, T.T.; Li, J.; Chandra, V.; Pervushin, K.; Beuerman, R.; Liu, S. Antimicrobial activity profiles of amphiphilic xanthone derivatives are a function of their molecular oligomerization. BBA-Biomembranes 2018, 860, 2281–2298. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.C. Synthesis of Caged Garcinia Xanthone Analogues; University of California: San Diego, CA, USA, 2009. [Google Scholar]
- Zhang, X.; Li, X.; Sun, H.; Jiang, Z.; Tao, L.; Gao, Y.; Guo, Q.; You, Q. Synthesis and evaluation of novel aza-caged garcinia xanthones. Org. Biomol. Chem. 2012, 10, 3288–3299. [Google Scholar] [CrossRef] [PubMed]
- Rewcastle, G.W.; Atwell, G.J.; Zhuang, L.; Baguley, B.C.; Denny, W.A. Potential antitumor agents. 61. Structure-activity relationships for in vivo colon 38 activity among disubstituted 9-oxo-9h-xanthene-4-acetic acids. J. Med. Chem. 1991, 34, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Baguley, B.C.; McKeage, M.J. Asa404: A tumor vascular-disrupting agent with broad potential for cancer therapy. Future Oncol. 2010, 6, 1537–1543. [Google Scholar] [CrossRef] [PubMed]
- Head, M.; Jameson, M.B. The development of the tumor vascular-disrupting agent asa404 (vadimezan, dmxaa): Current status and future opportunities. Expert Opin. Investig. Drugs 2010, 19, 295–304. [Google Scholar] [CrossRef]
- Rehman, F.; Rustin, G. Asa404: Update on drug development. Expert Opin. Investig. Drugs 2008, 17, 1547–1551. [Google Scholar] [CrossRef]
- Ching, L.M. Asa404. Vascular-disrupting agent, oncolytic. Drugs Future 2008, 33, 561–569. [Google Scholar] [CrossRef]
- McKeage, M.J.; Kelland, L.R. 5,6-dimethylxanthenone-4-acetic acid (dmxaa): Clinical potential in combination with taxane-based chemotherapy. Am. J. Cancer 2006, 5, 155–162. [Google Scholar] [CrossRef]
- Baguley, B.C.; Wilson, W.R. Potential of dmxaa combination therapy for solid tumors. Expert Rev. Anticanc. 2002, 2, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Baguley, B.C. Antivascular therapy of cancer: Dmxaa. Lancet. Oncol. 2003, 4, 141–148. [Google Scholar] [CrossRef]
- McKeage, M. Clinical trials of vascular disrupting agents in advanced non-small-cell lung cancer. Clin. Lung Cancer 2011, 12, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Kestell, P.; Baguley, B.C.; Paxton, J.W. 5,6-dimethylxanthenone-4-acetic acid (dmxaa): A new biological response modifier for cancer therapy. Invest. New Drugs 2002, 20, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Daei Farshchi Adli, A.; Jahanban-Esfahlan, R.; Seidi, K.; Samandari-Rad, S.; Zarghami, N. An overview on vadimezan (dmxaa): The vascular disrupting agent. Chem. Biol. Drug. Des. 2018, 91, 996–1006. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.S.; Brandao, P.; Fernandes, C.S.G.; da Silva, M.; de Sousa, M.; Pinto, M.M.M. Drug-like properties and adme of xanthone derivatives: The antechamber of clinical trials. Curr. Med. Chem. 2016, 23, 3654–3686. [Google Scholar] [CrossRef] [PubMed]
- Jameson, M.B.; Head, M. Pharmacokinetic evaluation of vadimezan (asa404, 5,6-dimethylxanthenone-4- acetic acid, dmxaa). Expert Opin. Drug Metab. Toxicol. 2011, 7, 1315–1326. [Google Scholar] [CrossRef] [PubMed]
- Shirey, K.A.; Nhu, Q.M.; Yim, K.C.; Roberts, Z.J.; Teijaro, J.R.; Farber, D.L.; Blanco, J.C.; Vogel, S.N. The anti-tumor agent, 5,6-dimethylxanthenone-4-acetic acid (dmxaa), induces ifn-β-mediated antiviral activity in vitro and in vivo. J. Leukoc. Biol. 2011, 89, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.H.; Zhang, Y.; Shen, J.; Zhang, S.; Chen, L.; Gu, J.; Mruk, J.S.; Cheng, G.; Zhu, L.; Kunapuli, S.P.; et al. Tumor vascular disrupting agent 5,6-dimethylxanthenone-4-acetic acid inhibits platelet activation and thrombosis via inhibition of thromboxane a2 signaling and phosphodiesterase. J. Thromb. Haemo. 2013, 11, 1855–1866. [Google Scholar]
- Rewcastle, G.W.; Atwell, G.J.; Baguley, B.C.; Boyd, M.; Thomsen, L.L.; Zhuang, L.; Denny, W.A. Potential antitumor agents. 63. Structure-activity relationships for side-chain analogs of the colon 38 active agent 9-oxo-9h-xanthene-4-acetic acid. J. Med. Chem. 1991, 34, 2864–2870. [Google Scholar] [CrossRef]
- Nakanishi, M.; Oe, T.; Tsuruda, M.; Matsuo, H.; Sakuragi, S. Studies on anti inflammatory agents. Xxxi. Studies on the synthesis and anti inflammatory activity of xanthenyl and benzo pyranopyridinylacetic acid derivatives (japanese). yakugaku zasshi 1976, 96, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Marona, H. [synthesis of some 2-xanthonylmethylthioalkanoic acids.]. Acta Pol. Pharm. 1988, 45, 13–34. [Google Scholar]
- Marona, H.; Pȩkala, E.; Gunia, A.; Czuba, Z.; Szneler, E.; Sadowski, T.; Król, W. The influence of some xanthone derivatives on the activity of j-774a.1 cells. Sci. Pharm. 2009, 77, 743–754. [Google Scholar] [CrossRef]
- Żelaszczyk, D.; Lipkowska, A.; Szkaradek, N.; Słoczyńska, K.; Gunia-Krzyżak, A.; Librowski, T.; Marona, H. Synthesis and preliminary anti-inflammatory evaluation of xanthone derivatives. Heterocycl. Commun. 2018, 24, 231–236. [Google Scholar] [CrossRef]
- Sousa, E.; Paiva, A.; Nazareth, N.; Gales, L.; Damas, A.M.; Nascimento, M.S.; Pinto, M. Bromoalkoxyxanthones as promising antitumor agents: Synthesis, crystal structure and effect on human tumor cell lines. Eur. J. Med. Chem. 2009, 44, 3830–3835. [Google Scholar] [CrossRef] [PubMed]
- Sousa, E.; Palmeira, A.; Cordeiro, A.S.; Sarmento, B.; Ferreira, D.; Lima, R.T.; Vasconcelos, M.H.; Pinto, M. Bioactive xanthones with effect on p-glycoprotein and prediction of intestinal absorption. Med. Chem. Res. 2013, 22, 2115–2123. [Google Scholar] [CrossRef]
- Cruz, I.; Puthongking, P.; Cravo, S.; Palmeira, A.; Cidade, H.; Pinto, M.; Sousa, E. Xanthone and flavone derivatives as dual agents with acetylcholinesterase inhibition and antioxidant activity as potential anti-alzheimer agents. J. Chem-Ny 2017, 2017. [Google Scholar] [CrossRef]
- Neves, M.P.; Cidade, H.; Pinto, M.; Silva, A.M.; Gales, L.; Damas, A.M.; Lima, R.T.; Vasconcelos, M.H.; de Sao Jose Nascimento, M. Prenylated derivatives of baicalein and 3,7-dihydroxyflavone: Synthesis and study of their effects on tumor cell lines growth, cell cycle and apoptosis. Eur. J. Med. Chem. 2011, 46, 2562–2574. [Google Scholar] [CrossRef]
- Paiva, A.M.; Sousa, M.E.; Camoes, A.; Nascimento, M.S.J.; Pinto, M.M.M. Prenylated xanthones: Antiproliferative effects and enhancement of the growth inhibitory action of 4-hydroxytamoxifen in estrogen receptor-positive breast cancer cell line. Med. Chem. Res. 2012, 21, 552–558. [Google Scholar] [CrossRef]
- Azevedo, C.M.; Afonso, C.M.; Soares, J.X.; Reis, S.; Sousa, D.; Lima, R.T.; Vasconcelos, M.H.; Pedro, M.; Barbosa, J.; Gales, L.; et al. Pyranoxanthones: Synthesis, growth inhibitory activity on human tumor cell lines and determination of their lipophilicity in two membrane models. Eur. J. Med. Chem. 2013, 69, 798–816. [Google Scholar] [CrossRef]
- Urbatzka, R.; Freitas, S.; Palmeira, A.; Almeida, T.; Moreira, J.; Azevedo, C.; Afonso, C.; Correia-da-Silva, M.; Sousa, E.; Pinto, M.; et al. Lipid reducing activity and toxicity profiles of a library of polyphenol derivatives. Eur. J. Med. Chem. 2018, 151, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.; Masawang, K.; Tiritan, M.E.; Sousa, E.; De Lima, V.; Afonso, C.; Bousbaa, H.; Sudprasert, W.; Pedro, M.; Pinto, M.M. New chiral derivatives of xanthones: Synthesis and investigation of enantioselectivity as inhibitors of growth of human tumor cell lines. Bioorg. Med. Chem. 2014, 22, 1049–1062. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.; Brandão, P.; Santos, A.; Tiritan, M.E.; Afonso, C.; Cass, Q.B.; Pinto, M.M. Resolution and determination of enantiomeric purity of new chiral derivatives of xanthones using polysaccharide-based stationary phases. J. Chromatogr. A 2012, 1269, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Arabanian, A.; Mohammadnejad, M.; Balalaie, S. A novel and efficient approach for the amidation of c-terminal peptides. J. Iran Chem. Soc. 2010, 7, 840–845. [Google Scholar] [CrossRef]
- Twibanire, J.D.K.; Grindley, T.B. Efficient and controllably selective preparation of esters using uronium-based coupling agents. Org. Lett. 2011, 13, 2988–2991. [Google Scholar] [CrossRef] [PubMed]
- Balalaie, S.; Mahdidoust, M.; Eshaghi-Najafabadi, R. 2-(1h-benzotriazole-1-yl)-1,1,3,3-tetramethyluronium tetrafluoro borate (tbtu) as an efficient coupling reagent for the esterification of carboxylic acids with alcohols and phenols at room temperature. Chin. J. Chem. 2008, 26, 1141–1144. [Google Scholar] [CrossRef]
- Balalaie, S.; Mahdidoust, M.; Eshaghi-Najafabadi, R. 2-(1h-benzotriazole-1-yl)-1, 1, 3, 3-tetramethyluronium tetrafluoroborate as an efficient coupling reagent for the amidation and phenylhydrazation of carboxylic acids at room temperature. J. Iran Chem. Soc. 2007, 4, 364–369. [Google Scholar] [CrossRef]
- Naik, S.A.; Lalithamba, H.S.; Sureshbabu, V.V. Application of 2-(1h-benzotriazol-1-yl)-1,1,3,3-tetramethyluronium tetrafluoroborate (tbtu) for the synthesis of acid azides. Indian J. Chem. Sect. 2011, 50, 103–109. [Google Scholar] [CrossRef]
- Fernandes, C.; Tiritan, M.E.; Cass, Q.; Kairys, V.; Fernandes, M.X.; Pinto, M. Enantioseparation and chiral recognition mechanism of new chiral derivatives of xanthones on macrocyclic antibiotic stationary phases. J. Chromatogr. A 2012, 1241, 60–68. [Google Scholar] [CrossRef]
- Phyo, Y.Z.; Cravo, S.; Palmeira, A.; Tiritan, M.E.; Kijjoa, A.; Pinto, M.M.M.; Fernandes, C. Enantiomeric resolution and docking studies of chiral xanthonic derivatives on chirobiotic columns. Molecules 2018, 23, 142. [Google Scholar] [CrossRef]
- Fernandes, C.; Palmeira, A.; Santos, A.; Tiritan, M.E.; Afonso, C.; Pinto, M.M. Enantioresolution of chiral derivatives of xanthones on (s,s)-whelk-o1 and l -phenylglycine stationary phases and chiral recognition mechanism by docking approach for (s,s)-whelk-o1. Chirality 2013, 25, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Carraro, M.L.; Palmeira, A.; Tiritan, M.E.; Fernandes, C.; Pinto, M.M.M. Resolution, determination of enantiomeric purity and chiral recognition mechanism of new xanthone derivatives on (s,s)-whelk-o1 stationary phase. Chirality 2017, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.; Pinto, M.M.M.; Tiritan, M.E. Enantioresolution of chiral derivatives of xanthones on different types of liquid chromatography stationary phases: a comparative study. Curr. Chromatogr. 2014, 1, 139–150. [Google Scholar] [CrossRef]
- Fernandes, C.; Palmeira, A.; Ramos, I.I.; Carneiro, C.; Afonso, C.; Tiritan, M.E.; Cidade, H.; Pinto, P.C.A.G.; Saraiva, M.L.M.F.S.; Reis, S.; et al. Chiral derivatives of xanthones: Investigation of the effect of enantioselectivity on inhibition of cyclooxygenases (cox-1 and cox-2) and binding interaction with human serum albumin. Pharmaceuticals 2017, 10, 50. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.; Oliveira, L.; Tiritan, M.E.; Leitao, L.; Pozzi, A.; Noronha-Matos, J.B.; Correia-de-Sá, P.; Pinto, M.M. Synthesis of new chiral xanthone derivatives acting as nerve conduction blockers in the rat sciatic nerve. Eur. J. Med. Chem. 2012, 55, 1–11. [Google Scholar] [CrossRef]
- Lopes, A.; Martins, E.; Silva, R.; Pinto, M.M.M.; Remião, F.; Sousa, E.; Fernandes, C. Chiral thioxanthones as modulators of p-glycoprotein: Synthesis and enantioselectivity studies. Molecules 2018, 23, 626. [Google Scholar] [CrossRef]
- Marona, H. Synthesis and anticonvulsant effects of some aminoalkanolic derivatives of xanthone. Pharmazie 1998, 53, 672–676. [Google Scholar]
- Marona, H. Evaluation of some 2-substituted derivatives of xanthone for anticonvulsant properties. Pharmazie 1998, 53, 405–409. [Google Scholar]
- Marona, H.; Gorka, Z.; Szneler, E. Aminoalkanolic derivatives of xanthone with potential antiepileptic activity. Pharmazie 1998, 53, 219–223. [Google Scholar]
- Marona, H.; Pękala, E.; Antkiewicz-Michaluk, L.; Walczak, M.; Szneler, E. Anticonvulsant activity of some xanthone derivatives. Bioorg. Med. Chem. 2008, 16, 7234–7244. [Google Scholar] [CrossRef]
- Marona, H.; Pekala, E.; Filipek, B.; Maciag, D.; Szneler, E. Pharmacological properties of some aminoalkanolic derivatives of xanthone. Pharmazie 2001, 56, 567. [Google Scholar] [PubMed]
- Marona, H.; Szkaradek, N.; Karczewska, E.; Trojanowska, D.; Budak, A.; Bober, P.; Przepiórka, W.; Cegla, M.; Szneler, E. Antifungal and antibacterial activity of the newly synthesized 2-xanthone derivatives. Archiv. Der. Pharmazie 2009, 342, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Marona, H.; Szkaradek, N.; Rapacz, A.; Filipek, B.; Dybała, M.; Siwek, A.; Cegła, M.; Szneler, E. Preliminary evaluation of pharmacological properties of some xanthone derivatives. Bioorg. Med. Chem. 2009, 17, 1345–1352. [Google Scholar] [CrossRef] [PubMed]
- Klesiewicz, K.; Żelaszczyk, D.; Trojanowska, D.; Bogusz, B.; Małek, M.; Waszkielewicz, A.; Szkaradek, N.; Karczewska, E.; Marona, H.; Budak, A. Preliminary antifungal activity assay of selected chlorine-containing derivatives of xanthone and phenoxyethyl amines. Chem. Biol. Drug. Des. 2018, 92, 1867–1875. [Google Scholar] [CrossRef] [PubMed]
- Waszkielewicz, A.M.; Słoczyńska, K.; Pękala, E.; Żmudzki, P.; Siwek, A.; Gryboś, A.; Marona, H. Design, synthesis, and anticonvulsant activity of some derivatives of xanthone with aminoalkanol moieties. Chem. Biol. Drug. Des. 2017, 89, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Klesiewicz, K.; Karczewska, E.; Budak, A.; Marona, H.; Szkaradek, N. Anti-helicobacter pylori activity of some newly synthesized derivatives of xanthone. J. Antibiot. 2016, 69, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Kubacka, M.; Szkaradek, N.; Mogilski, S.; Pańczyk, K.; Siwek, A.; Gryboś, A.; Filipek, B.; Żmudzki, P.; Marona, H.; Waszkielewicz, A.M. Design, synthesis and cardiovascular evaluation of some aminoisopropanoloxy derivatives of xanthone. Bioorg. Med. Chem. 2018, 26, 3773–3784. [Google Scholar] [CrossRef]
- Szkaradek, N.; Rapacz, A.; Pytka, K.; Filipek, B.; Zelaszczyk, D.; Szafrański, P.; Słoczyńska, K.; Marona, H. Cardiovascular activity of the chiral xanthone derivatives. Bioorg. Med. Chem. 2015, 23, 6714–6724. [Google Scholar] [CrossRef]
- Librowski, T.; Czarnecki, R.; Jastrzebska-Wiesek, M.; Opoka, W.; Marona, H. The influence of some aminoalkanolic xanthone derivatives on central nervous and cardiovascular systems in rodents. Boll. Chim. Farm 2004, 143, 267–274. [Google Scholar]
- Szkaradek, N.; Gunia, A.; Waszkielewicz, A.M.; Antkiewicz-Michaluk, L.; Cegła, M.; Szneler, E.; Marona, H. Anticonvulsant evaluation of aminoalkanol derivatives of 2-and 4-methylxanthone. Bioorg. Med. Chem. 2013, 21, 1190–1198. [Google Scholar] [CrossRef]
- Rajtar, G.; Zolkowska, D.; Kleinrok, Z.; Marona, H. Pharmacological properties of some xanthone derivatives. Acta Pol. Pharm. 1999, 56, 311–318. [Google Scholar] [PubMed]
- Jastrzêbska-Wiêsek, M.; Librowski, T.; Czarnecki, R.; Marona, H.; Nowak, G. Central activity of new xanthone derivatives with chiral center in some pharmacological tests in mice. Pol. J. Pharmacol. 2003, 55, 461–466. [Google Scholar] [PubMed]
- Jastrzebska-Wiesek, M.; Czarnecki, R.; Marona, H. The anticonvulsant, local anesthetic and hemodynamic properties of some chiral aminobutanol derivatives of xanthone. Acta Pol. Pharm. 2008, 65, 591–600. [Google Scholar] [PubMed]
- Librowski, T.; Czamecki, R.; Jastrzebska, M. Chiral 2-amino-1-butanol xanthone derivatives as potential antiarrhythmic and hypotensive agents. Acta Pol. Pharm. 1999, 56, 87–90. [Google Scholar] [PubMed]
- Rajtar, G.; Zolkowska, D.; Kleinrok, Z.; Marona, H. Antiplatelets activity of some xanthone derivatives. Acta Pol. Pharm. 1999, 56, 319–324. [Google Scholar] [PubMed]
- Słoczyńska, K.; Pękala, E.; Wajda, A.; Węgrzyn, G.; Marona, H. Evaluation of mutagenic and antimutagenic properties of some bioactive xanthone derivatives using vibrio harveyi test. Lett. Appli. Microbiol. 2010, 50, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Sypniewski, D.; Szkaradek, N.; Loch, T.; Waszkielewicz, A.M.; Gunia-Krzyżak, A.; Matczyńska, D.; Sołtysik, D.; Marona, H.; Bednarek, I. Contribution of reactive oxygen species to the anticancer activity of aminoalkanol derivatives of xanthone. Invest. New Drugs 2018, 36, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Leng, J.; Rakesh, K.P.; Darshini, N.; Shubhavathi, T.; Vivek, H.K.; Mallesha, N.; Qin, H.-L. Synthesis and molecular docking studies of xanthone attached amino acids as potential antimicrobial and anti-inflammatory agents. Med. Chem. Commun. 2017, 8, 1706–1719. [Google Scholar] [CrossRef] [PubMed]
- Rakesh, K.P.; Darshini, N.; Manukumar, H.M.; Vivek, H.K.; Prasanna, D.S.; Mallesha, N. Xanthone conjugated amino acids as potential anticancer and DNA binding agents: Molecular docking, cytotoxicity and sar studies. Anticancer Agents Med. Chem. 2018. [Google Scholar] [CrossRef] [PubMed]
- Szkaradek, N.; Stachura, K.; Waszkielewicz, A.; Cegla, M.; Szneler, E.; Marona, H. Synthesis and antimycobacterial assay of some xanthone derivatives. Acta Pol. Pharm. 2008, 65, 21–28. [Google Scholar] [PubMed]
- Marona, H.; Szkaradek, N.; Kubacka, M.; Bednarski, M.; Filipek, B.; Cegla, M.; Szneler, E. Synthesis and evaluation of some xanthone derivatives for anti-arrhythmic, hypotensive properties and their affinity for adrenergic receptors. Archiv. der Pharmazie 2008, 341, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Marona, H.; Librowski, T.; Cegła, M.; ERDOĞAN, C.; Sahin, N.O. Antiarrhythmic and antihypertensive activity of some xanthone derivatives. Acta Pol. Pharm. Drug Res. 2008, 65, 383–390. [Google Scholar]
- Szkaradek, N.; Rapacz, A.; Pytka, K.; Filipek, B.; Siwek, A.; Cegła, M.; Marona, H. Synthesis and preliminary evaluation of pharmacological properties of some piperazine derivatives of xanthone. Bioorg. Med. Chem. 2013, 21, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Waszkielewicz, A.; Gunia, A.; Szkaradek, N.; Pytka, K.; Siwek, A.; Satała, G.; Bojarski, A.; Szneler, E.; Marona, H. Synthesis and evaluation of pharmacological properties of some new xanthone derivatives with piperazine moiety. Bioorg. Med. Chem. Lett. 2013, 23, 4419–4423. [Google Scholar] [CrossRef] [PubMed]
- Rapacz, A.; Sapa, J.; Nowiński, L.; Mogilski, S.; Pytka, K.; Filipek, B.; Siwek, A.; Szkaradek, N.; Marona, H. Biofunctional studies of new 2-methoxyphenylpiperazine xanthone derivatives with α1-adrenolytic properties. Phytochem. Rep. 2015, 67, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Rapacz, A.; Sapa, J.; Pytka, K.; Dudek, M.; Filipek, B.; Szkaradek, N.; Marona, H. Antiarrhythmic activity of new 2-methoxyphenylpiperazine xanthone derivatives after ischemia/reperfusion in rats. Phytochem. Rep. 2015, 67, 1163–1167. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-W.; Kang, J.-J. Mechanism of vasorelaxation of thoracic aorta caused by xanthone. Eur. J. Pharmacol. 1997, 336, 23–28. [Google Scholar] [CrossRef]
- Cherkadu, V.; Kalavagunta, P.K.; Ningegowda, M.; Shivananju, N.S.; Madegowda, M.; Priya, B.S. Fecl3-catalyzed three-component one-pot synthesis of novel 4-[(benzo[d]thiazol-2-ylamino)(phenyl)methyl]-3-hydroxy-9h-xanthen-9-ones. Synlett 2016, 27, 1116–1120. [Google Scholar] [CrossRef]

























| CDXs | Biological Activities | Ref. |
|---|---|---|
| Kielcorin derivatives 2–9 | Antitumor and protein kinase C inhibition | [56,58,59,61] |
| Psorospermin derivatives 11–15 | Antitumor | [64] |
| Muchimangin derivatives 16–20 | Antimicrobial | [66] |
| Mangiferin derivatives 21–57 | Antipyretic, antimicrobial, analgesic, antioxidant, anti-inflammatory, antidiabetic, anticoagulant and antiplatelet | [68,72,73,75,76,77,78,81,82,83,86] |
| α-Mangostin derivatives 58–70 | Antimicrobial, hemolytic and antimycobacterial | [91,95,96,97,98,99,118] |
| Caged xanthones 71–155 | Antimalarial, antitumor, anti-proliferation and anti-angiogenesis | [106,108,109,111,113,114,115,116,117,119,120] |
| CDXs | Biological Activities | Ref. |
|---|---|---|
| XAA, DMXAA and analogues 156–164 | Antitumor, | [122,123,124,125,126,127,128,129,130,131,132,133,136] |
| antiviral, | [134] | |
| antiplatelet, antithrombotic, | [135] | |
| anti-inflammatory | [137] | |
| and analgesic | [140] | |
| Aminoalkanolic CDXs 165–243 | Cyclooxygenases inhibition, | [160] |
| antitumor, | [148] | |
| anticonvulsant, | [139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,167,171,175,176,177,178,179] | |
| cardiovascular, | [167,169,173,174,175,179,180] | |
| antiplatelet aggregation, | [181] | |
| antimutagenic, | [182] | |
| antifungal, | [170] | |
| antibacterial and | [172] | |
| anticancer | [183] | |
| CDXs conjugated with amines, amino esters and amino acids 244–263 | Anti-inflammatory | [168,172,186] |
| CDXs containing piperazine moieties and analogues 264–294 | Anticonvulsant, | [165] |
| antiplatelet aggregation, | [181] | |
| cardiovascular, | [173,187,188,189,190] | |
| antifungal and | [188] | |
| antibacterial | [172] | |
| CDXs containing other moieties 295–310 | No activities reported | [194] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, C.; Carraro, M.L.; Ribeiro, J.; Araújo, J.; Tiritan, M.E.; Pinto, M.M.M. Synthetic Chiral Derivatives of Xanthones: Biological Activities and Enantioselectivity Studies. Molecules 2019, 24, 791. https://doi.org/10.3390/molecules24040791
Fernandes C, Carraro ML, Ribeiro J, Araújo J, Tiritan ME, Pinto MMM. Synthetic Chiral Derivatives of Xanthones: Biological Activities and Enantioselectivity Studies. Molecules. 2019; 24(4):791. https://doi.org/10.3390/molecules24040791
Chicago/Turabian StyleFernandes, Carla, Maria Letícia Carraro, João Ribeiro, Joana Araújo, Maria Elizabeth Tiritan, and Madalena M. M. Pinto. 2019. "Synthetic Chiral Derivatives of Xanthones: Biological Activities and Enantioselectivity Studies" Molecules 24, no. 4: 791. https://doi.org/10.3390/molecules24040791
APA StyleFernandes, C., Carraro, M. L., Ribeiro, J., Araújo, J., Tiritan, M. E., & Pinto, M. M. M. (2019). Synthetic Chiral Derivatives of Xanthones: Biological Activities and Enantioselectivity Studies. Molecules, 24(4), 791. https://doi.org/10.3390/molecules24040791