Mechanosynthesis of Photochromic Oligophenyleneimines: Optical, Electrochemical and Theoretical Studies
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization
2.2. Optical Studies
2.2.1. Absorption Spectra in Different Solvents
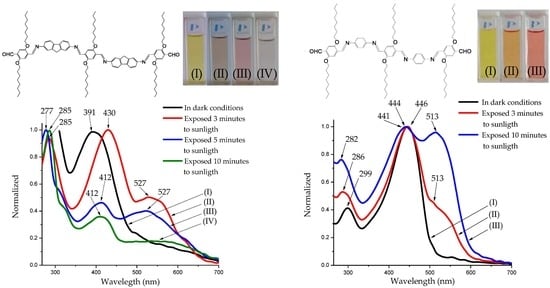
2.2.2. Photochromic Properties
2.3. Electrochemical Analysis
2.4. Theoretical Study
3. Experimental
3.1. General
3.2. Mechanochemistry
3.3. Electrochemical
3.4. Computational
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Barachevsky, V.A. Advances in photonics of organic photochromism. J. Photochem. Photobiol. A Chem. 2018, 354, 61–69. [Google Scholar] [CrossRef]
- Iwai, D.; Takeda, S.; Hino, N.; Sato, K. Projection screen reflectance control for high contrast display using photochromic compounds and UV LEDs. Opt. Express 2014, 22, 13492. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Suemori, K.; Uemura, S.; Hoshino, S.; Takada, N.; Kodzasa, T.; Kamata, T. Development of Field-Effect Transistor-Type Photorewritable Memory Using Photochromic Interface Layer. Jpn. J. Appl. Phys. 2010, 49, 04DK09. [Google Scholar] [CrossRef]
- Yam, V.W.-W.; Ko, C.-C.; Zhu, N. Photochromic and Luminescence Switching Properties of a Versatile Diarylethene-Containing 1,10-Phenanthroline Ligand and Its Rhenium(I) Complex. JACS Commun. 2004, 126, 12734–12735. [Google Scholar] [CrossRef]
- Thanopulos, I.; Kral, P.; Shapiro, M.; Paspalakis, E. Optical control of molecular switches. J. Mod. Opt. 2009, 56, 686–703. [Google Scholar] [CrossRef]
- Xiang, N.; Gao, Z.; Tian, G.; Chen, Y.; Liang, W.; Huang, J.; Dong, Q.; Wong, W.; Su, J. Novel fluorene/indole-based hole transport materials with high thermal stability for efficient OLEDs. Dyes Pigments 2017, 137, 36–42. [Google Scholar] [CrossRef]
- Fu, L.-N.; Leng, B.; Li, Y.-S.; Gao, X.-K. Photoresponsive organic field-effect transistors involving photochromic molecules. Chin. Chem. Lett. 2016, 27, 1319–1329. [Google Scholar] [CrossRef]
- Lutsyk, P.; Janus, K.; Sworakowski, J.J. Photoswitching of an n-Type Organic Field Effect Transistor by a Reversible Photochromic Reaction in the Dielectric Film. Phys. Chem. C 2011, 115, 3106–3114. [Google Scholar] [CrossRef]
- Wu, L.Y.L.; Zhao, Q.; Huang, H.; Lim, R.J. Sol-gel based photochromic coating for solar responsive smart window. Surf. Coat. Tech. 2017, 320, 601–607. [Google Scholar] [CrossRef]
- Spanu, A.; Viola, F.; Lai, S.; Coseddu, P.; Ricci, P.C.; Bonfiglio, A. A reference-less Ph sensor based on an organic field effect transistor with tunable sensitivity. Org. Electron. 2017, 48, 188–193. [Google Scholar] [CrossRef]
- Chen, C.H.; Lin, C.F.; Wang, K.H.; Liu, H.C.; Zan, H.W.; Meng, H.F.; Hortschitz, W.; Steiner, H.; Kainz, A.; Sauter, T. High-resolution proximity sensor flexible semi-transparent organic photo detector. Org. Electron. 2017, 49, 305–312. [Google Scholar] [CrossRef]
- Chuang, M.Y.; Lin, Y.T.; Tung, T.W.; Chang, L.Y.; Zan, H.W.; Meng, H.F.; Lu, C.J.; Tao, Y.T. Room-temperature-operated organic-based acetone gas sensor for breath analysis. Sens. Actuators B Chem. 2018, 260, 593–600. [Google Scholar] [CrossRef]
- Corrochano, D.R.; de la Hoz, A.; Sánchez-Migallón, A.M.; Caballero, R.; Ramírez, J.R. Synthesis of imine-derived triazines with Donor–Acceptor properties. J. Clean. Prod. 2016, 118, 223–228. [Google Scholar] [CrossRef]
- Li, Z.; Yin, J.; Wu, X.; Lin, Y.; Zeng, Q.; Fan, F.; Liu, S.H. Diarylethene-based imines and amines: Synthesis, photochromic properties and effects of substitution. J. Photochem. Photobiol. A Chem. 2011, 218, 192–198. [Google Scholar] [CrossRef]
- Martinez, A.I.; Coreño, O.; Cruz, J.; Vásquez, J.M.; Coreño, J.; Aleman, K.; Luna, G.; Pandiyan, T.; Vázques, R.A. Synthesis of Photochromic Oligophenylenimines: Optical and Computational Studies. Molecules 2015, 20, 5440–5455. [Google Scholar] [CrossRef] [Green Version]
- Berbasova, T.; Santos, E.M.; Nosrati, M.; Vasileiou, C.; Geiger, J.H.; Borhan, B. Light-Activated Reversible Imine Isomerization: Towards a Photochromic Protein Switch. ChemBioChem 2016, 17, 407–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coelho, P.J.; Castro, M.C.R.; Raposo, M.M.M. Reversible trans–cis photoisomerization of new pyrrolidene heterocyclic imines. J. Photochem. Photobiol. A Chem. 2013, 259, 59–65. [Google Scholar] [CrossRef]
- Keitaro, N.; Jonathan, P.; Pei, Y.; Rémi, M. Introduction: Organic Photochromic Molecules. In Photochromic Materials: Preparation, Properties and Applications, 1st ed.; Tian, H., Zhang, J., Eds.; Wiley-VCH: Weinheim, Germany, 2016; pp. 438–440. ISBN 978-3-527-33779-8. [Google Scholar]
- Luo, Y.; Utecht, M.; Dokić, J.; Korchak, S.; Vieth, H.-M.; Haag, R.; Saalfrank, P. cis-trans Isomerisation of Substituted Aromatic Imines: A Comparative Experimental and Theoretical Study. ChemPhysChem 2011, 12, 2311–2321. [Google Scholar] [CrossRef] [PubMed]
- Batista, R.M.F.; Costa, S.P.G.; Belsley, M.; Raposo, M.M.M. Synthesis and second-order nonlinear optical properties of new chromophores containing benzimidazole, thiophene, and pyrrole heterocycles. Tetrahedron 2007, 63, 9842–9849. [Google Scholar] [CrossRef] [Green Version]
- Liang, X.; Wang, K.; Zhang, R.; Li, K.; Lu, X.; Guo, K.; Wang, H.; Miao, Y.; Xu, H.; Wang, Z. Tetra-carbazole substituted spiro[fluorene-9,9′-xanthene]- based hole-transporting materials with high thermal stability and mobility for efficient OLEDS. Dyes Pigments 2017, 139, 764–771. [Google Scholar] [CrossRef]
- Zhao, S.; Li, Y.; Liu, C.; Zhao, Y. Recent advances in mechanochemical C–H functionalization reactions. Tetrahedron Lett. 2018, 59, 317–324. [Google Scholar] [CrossRef]
- Abdelwahab, H.; Hassan, S.; Yacout, G.; Mostafa, M.; El Sadek, M. Synthesis and Biological Evaluation of New Imine- and Amino-Chitosan Derivatives. Polymers 2015, 7, 2690–2700. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, C.M.; da Silva, D.L.; Modolo, L.V.; Alves, R.B.; de Resende, M.A.; Martins, C.V.B.; de Fátima, A. Schiff bases: A short review of their antimicrobial activities. J. Adv. Res. 2011, 2, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Ostroverkhova, O. Organic Optoelectronic Materials: Mechanisms and Applications. Chem. Rev. 2016, 116, 13279–13412. [Google Scholar] [CrossRef] [PubMed]
- Orgiu, E.; Samorì, P. 25th Anniversary Article: Organic Electronics Marries Photochromism: Generation of Multifunctional Interfaces, Materials, and Devices. Adv. Mater. 2014, 26, 1827–1845. [Google Scholar] [CrossRef] [PubMed]
- Tryznowski, M.; Tomczyk, K.; Fraś, Z.; Gregorowicz, J.; Rokicki, G.; Wawrzyńska, E.; Parzuchowski, P.G. Aliphatic Hyperbranched Polycarbonates: Synthesis, Characterization, and Solubility in Supercritical Carbon Dioxide. Macromolecules 2012, 45, 6819–6829. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, M.; Liu, Y.; Zhang, J.; Feng, S.; Xu, X.; Bo, Z. Simultaneous enhancement of the molecular planarity and the solubility of non-fullerene acceptors: Effect of aliphatic side-chain substitution on the photovoltaic performance. J. Mater. Chem. A 2017, 5, 7776–7783. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Y.; Hu, Y.; Ma, D.; Wang, L.; Jing, X.; Wang, F. Novel SolubleN-Phenyl-Carbazole-Containing PPVs for Light-Emitting Devices: Synthesis, Electrochemical, Optical, and Electroluminescent Properties. Macromol. Chem. Phys. 2004, 205, 247–255. [Google Scholar] [CrossRef]
- Sun, W.; Zhou, N.; Xiao, Y.; Wang, S.; Li, X. Novel carbazolyl-substituted spiro[acridine-9,9′-fluorene] derivatives as deep-blue emitting materials for OLED applications. Dyes Pigments 2018, 154, 30–37. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Cai, X.; Liu, K.; Dong, J.; Chang, S.; Su, S.-J. Spiro[fluorene-9,9′-thioxanthene] core based host materials for thermally activated delayed fluorescence devices. Dyes Pigments 2018. [Google Scholar] [CrossRef]
- Flores-Noria, R.; Vázquez, R.; Arias, E.; Moggio, I.; Rodríguez, M.; Ziolo, R.F.; Liebig, C. Synthesis and optoelectronic properties of phenylenevinylenequinoline macromolecules. New J. Chem. 2014, 38, 974. [Google Scholar] [CrossRef]
- Martin, R.L. Natural Transition Orbitals. J. Chem. Phys. 2003, 118, 4775. [Google Scholar] [CrossRef]
- Eilmes, A. Solvatochromic Probe in Molecular Solvents: Implicit versus Explicit Solvent Model. Theor. Chem. Acc. 2014, 9, 133. [Google Scholar] [CrossRef]
- Aradi, B.; Hourahine, B.; Frauenheim, T. DFTB+, a sparse matrix-based implementation of the DFTB method. J. Phys. Chem. A 2007, 111, 5678. [Google Scholar] [CrossRef] [PubMed]
- O’Boyle, N.M.; Tenderholt, A.L.; Langner, K.M. cclib: A library for package-independent computational chemistry algorithms. J. Comp. Chem. 2008, 29, 839. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision A.02; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |








| DAFCHO | FDACHO | |||||
|---|---|---|---|---|---|---|
| Solvents | λ max (nm) | ε × 103 (M−1 cm−1) | Eg (eV) | λ max (nm) | ε × 103 (M−1 cm−1) | Eg (eV) |
| Chloroform | 391 | 9.06 | 2.50 | 446 | 25.60 | 2.41 |
| Tetrahydrofuran | 441 | 16.69 | 2.46 | 439 | 13.62 | 2.43 |
| Toluene | 412 | 17.01 | 2.47 | 435 | 20.11 | 2.43 |
| Acetonitrile | 412 | 4.53 | 2.56 | 412 | 2.37 | 2.55 |
| OLIGOIMINE | Eox V | −Ered V | HOMO eV | LUMO eV | Eg(EC) eV |
|---|---|---|---|---|---|
| DAFCHO | 1.07 | 1.26 | 3.54 | 5.87 | 2.35 |
| FDACHO | 0.96 | 1.10 | 3.69 | 5.75 | 2.06 |
| Transition | Energy (eV) | Wavelength (nm) | Osc. Strength | Major Contribs |
|---|---|---|---|---|
| DAFCHO | ||||
| 2 | 3.15 | 393.89 | 1.2490 | H−1→LUMO (13%) HOMO→L + 2 (51%) |
| 17 | 4.24 | 292.55 | 0.4089 | H−2→LUMO (31%) |
| 16 | 4.20 | 295.18 | 0.3828 | H−2→LUMO (32%) |
| 27 | 4.73 | 262.11 | 0.3215 | H−1→L + 4 (31%) |
| 26 | 4.72 | 262.66 | 0.2376 | H−11→L + 1 (11%) H−10→L + 1 (13%) H−1→L + 4 (20%) |
| 1 | 3.02 | 410.38 | 0.2316 | H−1→LUMO (56%) HOMO→L + 2 (14%) |
| 4 | 3.40 | 364.57 | 0.2299 | H−4→L + 1 (14%) H−3→LUMO (21%) H−3→L + 1 (36%) |
| FDACHO | ||||
| 1 | 2.80 | 443.16 | 1.6216 | H−1→L + 1 (12%) HOMO→LUMO (62%) |
| 2 | 3.02 | 410.28 | 0.7902 | H−1→LUMO (53%) HOMO→L + 1 (19%) |
| 3 | 3.22 | 385.28 | 0.4051 | H−1→L + 1 (17%) H−1→L + 2 (16%) HOMO→L + 1 (22%) HOMO→L + 2 (13%) |
| 11 | 4.16 | 297.49 | 0.4037 | H−14→LUMO (11%) H−9→LUMO (19%) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amado-Briseño, M.A.; Zárate-Hernández, L.Á.; Alemán-Ayala, K.; Coreño Alonso, O.; Cruz-Borbolla, J.; Vásquez-Pérez, J.M.; Reyes-Cruz, V.E.; Veloz-Rodríguez, M.A.; Rueda-Soriano, E.; Pandiyan, T.; et al. Mechanosynthesis of Photochromic Oligophenyleneimines: Optical, Electrochemical and Theoretical Studies. Molecules 2019, 24, 849. https://doi.org/10.3390/molecules24050849
Amado-Briseño MA, Zárate-Hernández LÁ, Alemán-Ayala K, Coreño Alonso O, Cruz-Borbolla J, Vásquez-Pérez JM, Reyes-Cruz VE, Veloz-Rodríguez MA, Rueda-Soriano E, Pandiyan T, et al. Mechanosynthesis of Photochromic Oligophenyleneimines: Optical, Electrochemical and Theoretical Studies. Molecules. 2019; 24(5):849. https://doi.org/10.3390/molecules24050849
Chicago/Turabian StyleAmado-Briseño, Miguel Angel, Luis Ángel Zárate-Hernández, Karina Alemán-Ayala, Oscar Coreño Alonso, Julián Cruz-Borbolla, José Manuel Vásquez-Pérez, Víctor Esteban Reyes-Cruz, María Aurora Veloz-Rodríguez, Esteban Rueda-Soriano, Thangarasu Pandiyan, and et al. 2019. "Mechanosynthesis of Photochromic Oligophenyleneimines: Optical, Electrochemical and Theoretical Studies" Molecules 24, no. 5: 849. https://doi.org/10.3390/molecules24050849
APA StyleAmado-Briseño, M. A., Zárate-Hernández, L. Á., Alemán-Ayala, K., Coreño Alonso, O., Cruz-Borbolla, J., Vásquez-Pérez, J. M., Reyes-Cruz, V. E., Veloz-Rodríguez, M. A., Rueda-Soriano, E., Pandiyan, T., & Vázquez-García, R. A. (2019). Mechanosynthesis of Photochromic Oligophenyleneimines: Optical, Electrochemical and Theoretical Studies. Molecules, 24(5), 849. https://doi.org/10.3390/molecules24050849