Application of Antimicrobial Nanoparticles in Dentistry
Abstract
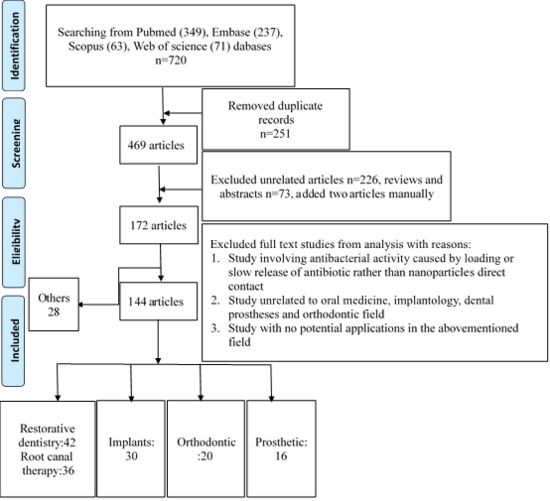
:1. Introduction
2. Antimicrobial Applications in Dentistry
2.1. Antimicrobial Activity in Oral Medicine
2.1.1. Restorative Dentistry
2.1.2. Root Canal Therapy
2.2. Implants Modified with Antibacterial Nanoparticles
2.3. Orthodontics
2.4. Other Applications
2.4.1. Antimicrobial Application in Prosthetic Fields
2.4.2. Periodontics and Preventive Medicine
3. Antibacterial Mechanism
4. Toxicity
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kasraei, S.; Sami, L. Antibacterial properties of composite resins incorporating silver and zinc oxide nanoparticles on Streptococcus mutans and Lactobacillus. Restor. Dent. Endod. 2014, 39, 109–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes, G.L.; Delbem, A.C.B. Nanosynthesis of Silver-Calcium Glycerophosphate: Promising Association against Oral Pathogens. Antibiotics (Basel) 2018, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Saafan, A.; Zaazou, M.H. Assessment of Photodynamic Therapy and Nanoparticles Effects on Caries Models. Open Access Maced. J. Med. Sci. 2018, 6, 1289–1295. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Zhang, Y. Development of a novel resin-based dental material with dual biocidal modes and sustained release of Ag+ ions based on photocurable core-shell AgBr/cationic polymer nanocomposites. J. Mater. Sci. Mater. Med. 2017, 28. [Google Scholar] [CrossRef] [PubMed]
- Magalhaes, A.P.; Moreira, F.C. Silver nanoparticles in resin luting cements: Antibacterial and physiochemical properties. J. Clin. Exp. Dent. 2016, 8, e415–e422. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Zhang, Y. Novel resin-based dental material with anti-biofilm activity and improved mechanical property by incorporating hydrophilic cationic copolymer functionalized nanodiamond. J. Mater. Sci. Mater. Med. 2018, 29, 162. [Google Scholar] [CrossRef]
- Xie, X.; Wang, L. Novel dental adhesive with triple benefits of calcium phosphate recharge, protein-repellent and antibacterial functions. Dent. Mater. 2017, 33, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, M.F.; Malaquias, P. The role of copper nanoparticles in an etch-and-rinse adhesive on antimicrobial activity, mechanical properties and the durability of resin-dentine interfaces. J. Dent. 2017, 61, 12–20. [Google Scholar] [CrossRef]
- Alberto Perez-Diaz, M.; Boegli, L. Silver nanoparticles with antimicrobial activities against Streptococcus mutans and their cytotoxic effect. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 55, 360–366. [Google Scholar] [CrossRef]
- Cai, Y.; Stromme, M. Photocatalytic inactivation of biofilms on bioactive dental adhesives. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 62–67. [Google Scholar] [CrossRef]
- Sabatini, C.; Mennito, A.S. Incorporation of bactericidal poly-acrylic acid modified copper iodide particles into adhesive resins. J. Dent. 2015, 43, 546–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cataldi, A.; Gallorini, M. Adhesion of human gingival fibroblasts/Streptococcus mitis co-culture on the nanocomposite system Chitlac-nAg. J. Mater. Sci. Mater. Med. 2016, 27, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, L.; Liu, Y. Nanocatalysts promote Streptococcus mutans biofilm matrix degradation and enhance bacterial killing to suppress dental caries in vivo. Biomaterials 2016, 101, 272–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutierrez, M.F.; Malaquias, P. Mechanical and microbiological properties and drug release modeling of an etch-and-rinse adhesive containing copper nanoparticles. Dent. Mater. 2017, 33, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Naha, P.C. Topical ferumoxytol nanoparticles disrupt biofilms and prevent tooth decay in vivo via intrinsic catalytic activity. Nat. Commun. 2018, 9. [Google Scholar] [CrossRef]
- Elgamily, H.M.; El-Sayed, H.S. The Antibacterial Effect of Two Cavity Disinfectants against One of Cariogenic Pathogen: An In vitro Comparative Study. Contemp. Clin. Dent. 2018, 9, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Esteban Florez, F.L.; Hiers, R.D. Antibacterial dental adhesive resins containing nitrogen-doped titanium dioxide nanoparticles. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 93, 931–943. [Google Scholar] [CrossRef]
- Andrade, V.; Martinez, A. Antibacterial activity against Streptococcus mutans and diametrical tensile strength of an interim cement modified with zinc oxide nanoparticles and terpenes: An in vitro study. J. Prosthet. Dent. 2018, 119, 862.e1–862.e7. [Google Scholar] [CrossRef]
- Dutra-Correa, M.; Leite, A. Antibacterial effects and cytotoxicity of an adhesive containing low concentration of silver nanoparticles. J. Dent. 2018, 77, 66–71. [Google Scholar] [CrossRef]
- Hamilton, M.F.; Otte, A.D. Physicomechanical and antibacterial properties of experimental resin-based dental sealants modified with nylon-6 and chitosan nanofibers. J. Biomed. Mater. Res. B Appl. Biomater. 2015, 103, 1560–1568. [Google Scholar] [CrossRef]
- Gerasymchuk, Y.; Lukowiak, A. New photosensitive nanometric graphite oxide composites as antimicrobial material with prolonged action. J. Inorg. Biochem. 2016, 159, 142–148. [Google Scholar] [CrossRef]
- Karasenkov, Y.; Frolov, G. Colloidal metal oxide nanoparticle systems: The new promising way to prevent antibiotic resistance during treatment of local infectious processes. In 3rd International Youth Conference on Interdisciplinary Problems of Nanotechnology, Biomedicine and Nanotoxicology; Refsnes, M., Gusev, A., Godymchuk, A., Bogdan, A., Eds.; IOP Conference Series-Materials Science and Engineering; IOP Publishing Ltd: London, UK, 2015. [Google Scholar]
- Paiva, L.; Fidalgo, T.K.S. Antibacterial properties and compressive strength of new one-step preparation silver nanoparticles in glass ionomer cements (NanoAg-GIC). J. Dent. 2018, 69, 102–109. [Google Scholar] [CrossRef]
- Renne, W.G.; Lindner, A. Antibacterial properties of copper iodide-doped glass ionomer-based materials and effect of copper iodide nanoparticles on collagen degradation. Clin. Oral Investig. 2017, 21, 369–379. [Google Scholar] [CrossRef]
- Garcia-Contreras, R.; Scougall-Vilchis, R.J. Mechanical, antibacterial and bond strength properties of nano-titanium-enriched glass ionomer cement. J. Appl. Oral Sci. 2015, 23, 321–328. [Google Scholar] [CrossRef] [Green Version]
- Hosida, T.Y.; Delbem, A.C.B. Ion release, antimicrobial and physio-mechanical properties of glass ionomer cement containing micro or nanosized hexametaphosphate, and their effect on enamel demineralization. Clin. Oral Investig. 2018. [Google Scholar] [CrossRef]
- Garcia, P.; Cardia, M.F.B. Antibacterial activity of glass ionomer cement modified by zinc oxide nanoparticles. Microsc. Res. Tech. 2017, 80, 456–461. [Google Scholar] [CrossRef]
- Pietrokovski, Y.; Nisimov, I. Antibacterial effect of composite resin foundation material incorporating quaternary ammonium polyethyleneimine nanoparticles. J. Prosthet. Dent. 2016, 116, 603–609. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, K. One-year water-ageing of calcium phosphate composite containing nano-silver and quaternary ammonium to inhibit biofilms. Int. J. Oral Sci. 2016, 8, 172–181. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Zhou, H. Effect of dimethylaminohexadecyl methacrylate mass fraction on fracture toughness and antibacterial properties of CaP nanocomposite. J. Dent. 2015, 43, 1539–1546. [Google Scholar] [CrossRef] [Green Version]
- Yue, S.; Wu, J. Novel dental adhesive resin with crack self-healing, antimicrobial and remineralization properties. J. Dent. 2018, 75, 48–57. [Google Scholar] [CrossRef]
- Zhang, N.; Melo, M.A. Development of a multifunctional adhesive system for prevention of root caries and secondary caries. Dent. Mater. 2015, 31, 1119–1131. [Google Scholar] [CrossRef] [Green Version]
- Xie, X.; Wang, L. Protein-repellent and antibacterial functions of a calcium phosphate rechargeable nanocomposite. J. Dent. 2016, 52, 15–22. [Google Scholar] [CrossRef]
- Al-Dulaijan, Y.A.; Cheng, L. Novel rechargeable calcium phosphate nanocomposite with antibacterial activity to suppress biofilm acids and dental caries. J. Dent. 2018, 72, 44–52. [Google Scholar] [CrossRef]
- Ibrahim, M.S.; AlQarni, F.D. Tuning Nano-Amorphous Calcium Phosphate Content in Novel Rechargeable Antibacterial Dental Sealant. Materials (Basel) 2018, 11, 1544. [Google Scholar] [CrossRef]
- Li, F.; Weir, M.D. Effect of salivary pellicle on antibacterial activity of novel antibacterial dental adhesives using a dental plaque microcosm biofilm model. Dent. Mater. 2014, 30, 182–191. [Google Scholar] [CrossRef]
- Aliasghari, A.; Khorasgani, M.R. Evaluation of antibacterial efficiency of chitosan and chitosan nanoparticles on cariogenic streptococci: An in vitro study. Iran. J. Microbiol. 2016, 8, 93–100. [Google Scholar]
- Wassel, M.O.; Khattab, M.A. Antibacterial activity against Streptococcus mutans and inhibition of bacterial induced enamel demineralization of propolis, miswak, and chitosan nanoparticles based dental varnishes. J. Adv. Res. 2017, 8, 387–392. [Google Scholar] [CrossRef]
- Covarrubias, C.; Trepiana, D. Synthesis of hybrid copper-chitosan nanoparticles with antibacterial activity against cariogenic Streptococcus mutans. Dent. Mater. J. 2018, 37, 379–384. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Weir, M.D. Development of novel self-healing and antibacterial dental composite containing calcium phosphate nanoparticles. J. Dent. 2015, 43, 317–326. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Hu, X. Novel magnetic nanoparticle-containing adhesive with greater dentin bond strength and antibacterial and remineralizing capabilities. Dent. Mater. 2018, 34, 1310–1322. [Google Scholar] [CrossRef]
- Chen, C.; Weir, M.D. Antibacterial activity and ion release of bonding agent containing amorphous calcium phosphate nanoparticles. Dent. Mater. 2014, 30, 891–901. [Google Scholar] [CrossRef] [Green Version]
- Nabavizadeh, M.; Abbaszadegan, A. Antibiofilm efficacy of positively charged imidazolium-based silver nanoparticles in Enterococcus faecalis using quantitative real-time PCR. Jundishapur J. Microbiol. 2017, 10. [Google Scholar] [CrossRef]
- Fan, W.; Wu, Y. Substantivity of Ag-Ca-Si mesoporous nanoparticles on dentin and its ability to inhibit Enterococcus faecalis. J. Mater. Sci. Mater. Med. 2016, 27. [Google Scholar] [CrossRef]
- Bruniera, J.F.; Silva-Sousa, Y.T. Development of intracanal formulation containing silver nanoparticles. Braz. Dent. J. 2014, 25, 302–306. [Google Scholar] [CrossRef]
- Martinez-Andrade, J.M.; Avalos-Borja, M. Dual function of EDTA with silver nanoparticles for root canal treatment-A novel modification. PLoS ONE 2018, 13, e0190866. [Google Scholar] [CrossRef]
- Kesler Shvero, D.; Zaltsman, N. Lethal bacterial trap: Cationic surface for endodontic sealing. J. Biomed. Mater. Res. A 2016, 104, 427–434. [Google Scholar] [CrossRef]
- Abbaszadegan, A.; Nabavizadeh, M. Positively charged imidazolium-based ionic liquid-protected silver nanoparticles: A promising disinfectant in root canal treatment. Int. Endod. J. 2015, 48, 790–800. [Google Scholar] [CrossRef]
- Shrestha, A.; Kishen, A. Antibiofilm efficacy of photosensitizer-functionalized bioactive nanoparticles on multispecies biofilm. J. Endod. 2014, 40, 1604–1610. [Google Scholar] [CrossRef]
- Shrestha, A.; Hamblin, M.R. Photoactivated rose bengal functionalized chitosan nanoparticles produce antibacterial/biofilm activity and stabilize dentin-collagen. Nanomedicine 2014, 10, 491–501. [Google Scholar] [CrossRef]
- Charannya, S.; Duraivel, D. Comparative Evaluation of Antimicrobial Efficacy of Silver Nanoparticles and 2% Chlorhexidine Gluconate When Used alone and in Combination Assessed Using Agar Diffusion Method: An In vitro Study. Contemp. Clin. Dent. 2018, 9, S204–S209. [Google Scholar] [CrossRef]
- Halkai, K.R.; Mudda, J.A. Evaluation of Antibacterial Efficacy of Fungal-Derived Silver Nanoparticles against Enterococcus faecalis. Contemp. Clin. Dent. 2018, 9, 45–48. [Google Scholar] [CrossRef]
- Halkai, K.R.; Mudda, J.A. Antibacterial Efficacy of Biosynthesized Silver Nanoparticles against Enterococcus faecalis Biofilm: An in vitro Study. Contemp. Clin. Dent. 2018, 9, 237–241. [Google Scholar] [CrossRef]
- Halkai, K.R.; Mudda, J.A. Evaluation of antibacterial efficacy of biosynthesized silver nanoparticles derived from fungi against endo-perio pathogens Porphyromonas gingivalis, Bacillus pumilus, and Enterococcus faecalis. J. Conserv. Dent. 2017, 20, 398–404. [Google Scholar] [CrossRef]
- Afkhami, F.; Akbari, S. Entrococcus faecalis Elimination in Root Canals Using Silver Nanoparticles, Photodynamic Therapy, Diode Laser, or Laser-activated Nanoparticles: An In Vitro Study. J. Endod. 2017, 43, 279–282. [Google Scholar] [CrossRef]
- Chavez-Andrade, G.M.; Tanomaru-Filho, M. Cytotoxicity, genotoxicity and antibacterial activity of poly(vinyl alcohol)-coated silver nanoparticles and farnesol as irrigating solutions. Arch. Oral Biol. 2017, 84, 89–93. [Google Scholar] [CrossRef]
- Monzavi, A.; Eshraghi, S. In vitro and ex vivo antimicrobial efficacy of nano-MgO in the elimination of endodontic pathogens. Clin. Oral Investig. 2015, 19, 349–356. [Google Scholar] [CrossRef]
- Del Carpio-Perochena, A.; Bramante, C.M. Chelating and antibacterial properties of chitosan nanoparticles on dentin. Restor. Dent. Endod. 2015, 40, 195–201. [Google Scholar] [CrossRef] [Green Version]
- Bukhari, S.; Kim, D. Novel Endodontic Disinfection Approach Using Catalytic Nanoparticles. J. Endod. 2018, 44, 806–812. [Google Scholar] [CrossRef]
- Zheng, T.; Huang, X. A liquid crystalline precursor incorporating chlorhexidine acetate and silver nanoparticles for root canal disinfection. Biomater. Sci. 2018, 6, 596–603. [Google Scholar] [CrossRef]
- Rodrigues, C.T.; de Andrade, F.B. Antibacterial properties of silver nanoparticles as a root canal irrigant against Enterococcus faecalis biofilm and infected dentinal tubules. Int. Endod. J. 2018, 51, 901–911. [Google Scholar] [CrossRef]
- Schwass, D.R.; Lyons, K.M. Antimicrobial Activity of a Colloidal AgNP Suspension Demonstrated In Vitro against Monoculture Biofilms: Toward a Novel Tooth Disinfectant for Treating Dental Caries. Adv. Dent. Res. 2018, 29, 117–123. [Google Scholar] [CrossRef]
- Wu, D.; Fan, W. Evaluation of the antibacterial efficacy of silver nanoparticles against Enterococcus faecalis biofilm. J. Endod. 2014, 40, 285–290. [Google Scholar] [CrossRef]
- Guerreiro Tanomaru, J.M.; Storto, I. Radiopacity, pH and antimicrobial activity of Portland cement associated with micro- and nanoparticles of zirconium oxide and niobium oxide. Dent. Mater. J. 2014, 33, 466–470. [Google Scholar] [CrossRef] [Green Version]
- Guerreiro-Tanomaru, J.M.; Trindade-Junior, A. Effect of Zirconium Oxide and Zinc Oxide Nanoparticles on Physicochemical Properties and Antibiofilm Activity of a Calcium Silicate-Based Material. Sci. World J. 2014, 2014. [Google Scholar] [CrossRef]
- Wang, L.; Xie, X. Novel bioactive root canal sealer to inhibit endodontic multispecies biofilms with remineralizing calcium phosphate ions. J. Dent. 2017, 60, 25–35. [Google Scholar] [CrossRef]
- Seung, J.; Weir, M.D. A Modified Resin Sealer: Physical and Antibacterial Properties. J. Endod. 2018, 44, 1553–1557. [Google Scholar] [CrossRef]
- Del Carpio-Perochena, A.; Kishen, A. Antibacterial Properties of Chitosan Nanoparticles and Propolis Associated with Calcium Hydroxide against Single- and Multispecies Biofilms: An In Vitro and In Situ Study. J. Endod. 2017, 43, 1332–1336. [Google Scholar] [CrossRef]
- Del Carpio-Perochena, A.; Kishen, A. Antibacterial Properties Associated with Chitosan Nanoparticle Treatment on Root Dentin and 2 Types of Endodontic Sealers. J. Endod. 2015, 41, 1353–1358. [Google Scholar] [CrossRef]
- Louwakul, P.; Saelo, A. Efficacy of calcium oxide and calcium hydroxide nanoparticles on the elimination of Enterococcus faecalis in human root dentin. Clin. Oral Investig. 2017, 21, 865–871. [Google Scholar] [CrossRef]
- Elshinawy, M.I.; Al-Madboly, L.A. Synergistic Effect of Newly Introduced Root Canal Medicaments; Ozonated Olive Oil and Chitosan Nanoparticles, Against Persistent Endodontic Pathogens. Front. Microbiol. 2018, 9, 1371. [Google Scholar] [CrossRef]
- Afkhami, F.; Pourhashemi, S.J. Antibiofilm efficacy of silver nanoparticles as a vehicle for calcium hydroxide medicament against Enterococcus faecalis. J. Dent. 2015, 43, 1573–1579. [Google Scholar] [CrossRef]
- Javidi, M.; Afkhami, F. Efficacy of a combined nanoparticulate/calcium hydroxide root canal medication on elimination of Enterococcus faecalis. Aust. Endod. J. 2014, 40, 61–65. [Google Scholar] [CrossRef]
- Vazquez-Garcia, F.; Tanomaru-Filho, M. Effect of Silver Nanoparticles on Physicochemical and Antibacterial Properties of Calcium Silicate Cements. Braz. Dent. J. 2016, 27, 508–514. [Google Scholar] [CrossRef] [Green Version]
- Nam, K.Y. Characterization and antimicrobial efficacy of Portland cement impregnated with silver nanoparticles. J. Adv. Prosthodont. 2017, 9, 217–223. [Google Scholar] [CrossRef] [Green Version]
- Abramovitz, I.; Wisblech, D. Intratubular Antibacterial Effect of Polyethyleneimine Nanoparticles: An Ex Vivo Study in Human Teeth. J. Nanomater. 2015. [Google Scholar] [CrossRef]
- Bahador, A.; Pourakbari, B. In vitro evaluation of the antimicrobial activity of nanosilver-mineral trioxide aggregate against frequent anaerobic oral pathogens by a membrane-enclosed immersion test. Biomed. J. 2015, 38, 77–83. [Google Scholar] [CrossRef]
- Jonaidi-Jafari, N.; Izadi, M. The effects of silver nanoparticles on antimicrobial activity of ProRoot mineral trioxide aggregate (MTA) and calcium enriched mixture (CEM). J. Clin. Exp. Dent. 2016, 8, e22–e26. [Google Scholar] [CrossRef] [Green Version]
- Barros, J.; Silva, M.G. Antibiofilm effects of endodontic sealers containing quaternary ammonium polyethylenimine nanoparticles. J. Endod. 2014, 40, 1167–1171. [Google Scholar] [CrossRef]
- Li, P.; Tong, Z. Antibacterial and biological properties of biofunctionalized nanocomposites on titanium for implant application. J. Biomater. Appl. 2016, 31, 205–214. [Google Scholar] [CrossRef]
- Wiedmer, D.; Petersen, F.C. Antibacterial effect of hydrogen peroxide-titanium dioxide suspensions in the decontamination of rough titanium surfaces. Biofouling 2017, 33, 451–459. [Google Scholar] [CrossRef]
- Yang, Y.; Ren, S. Safety and efficacy of PLGA(Ag-Fe3O4)-coated dental implants in inhibiting bacteria adherence and osteogenic inducement under a magnetic field. Int. J. Nanomed. 2018, 13, 3751–3762. [Google Scholar] [CrossRef]
- Rosenbaum, J.; Versace, D.L. Antibacterial properties of nanostructured Cu-TiO2 surfaces for dental implants. Biomater. Sci. 2017, 5, 455–462. [Google Scholar] [CrossRef]
- Shen, X.T.; Zhang, Y.Z. Effects on cytotoxicity and antibacterial properties of the incorporations of silver nanoparticles into the surface coating of dental alloys. J. Zhejiang Univ. Sci. B 2017, 18, 615–625. [Google Scholar] [CrossRef]
- Divakar, D.D.; Jastaniyah, N.T. Enhanced antimicrobial activity of naturally derived bioactive molecule chitosan conjugated silver nanoparticle against dental implant pathogens. Int. J. Biol. Macromol. 2018, 108, 790–797. [Google Scholar] [CrossRef]
- Jin, J.; Zhang, L. Ti-GO-Ag nanocomposite: The effect of content level on the antimicrobial activity and cytotoxicity. Int. J. Nanomed. 2017, 12, 4209–4224. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Su, P. Synthesis of TiO2 nanotubes with ZnO nanoparticles to achieve antibacterial properties and stem cell compatibility. Nanoscale 2014, 6, 9050–9062. [Google Scholar] [CrossRef]
- Massa, M.A.; Covarrubias, C. Synthesis of new antibacterial composite coating for titanium based on highly ordered nanoporous silica and silver nanoparticles. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 45, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, V.H.; Igai, F. Use of Silver Nanoparticles Reduces Internal Contamination of External Hexagon Implants by Candida albicans. Braz. Dent. J. 2015, 26, 458–462. [Google Scholar] [CrossRef]
- Weng, S.; Zhao, X. Synthesis, characterization, antibacterial activity in dark and in vitro cytocompatibility of Ag-incorporated TiO2 microspheres with high specific surface area. J. Mater. Sci. Mater. Med. 2018, 29, 50. [Google Scholar] [CrossRef] [PubMed]
- Pokrowiecki, R.; Zareba, T. In vitro studies of nanosilver-doped titanium implants for oral and maxillofacial surgery. Int. J. Nanomed. 2017, 12, 4285–4297. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Gan, K. Antibacterial properties of nano-silver coated PEEK prepared through magnetron sputtering. Dent. Mater. 2017, 33, e348–e360. [Google Scholar] [CrossRef] [PubMed]
- Gyorgyey, A.; Janovak, L. Investigation of the in vitro photocatalytic antibacterial activity of nanocrystalline TiO2 and coupled TiO2/Ag containing copolymer on the surface of medical grade titanium. J. Biomater. Appl. 2016, 31, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Cao, H. Hierarchical micro/nanostructured titanium with balanced actions to bacterial and mammalian cells for dental implants. Int. J. Nanomed. 2015, 10, 6659–6674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vilarrasa, J.; Delgado, L.M. In vitro evaluation of a multispecies oral biofilm over antibacterial coated titanium surfaces. J. Mater. Sci. Mater. Med. 2018, 29, 164. [Google Scholar] [CrossRef]
- Chen, P.; Wu, Z. Fabrication of a silver nanoparticle-coated collagen membrane with anti-bacterial and anti-inflammatory activities for guided bone regeneration. Biomed. Mater. 2018, 13. [Google Scholar] [CrossRef]
- Memarzadeh, K.; Sharili, A.S. Nanoparticulate zinc oxide as a coating material for orthopedic and dental implants. J. Biomed. Mater. Res. A 2015, 103, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Abdulkareem, E.H.; Memarzadeh, K. Anti-biofilm activity of zinc oxide and hydroxyapatite nanoparticles as dental implant coating materials. J. Dent. 2015, 43, 1462–1469. [Google Scholar] [CrossRef]
- Westas, E.; Hayashi, M. Bactericidal effect of photocatalytically-active nanostructured TiO2 surfaces on biofilms of the early oral colonizer, Streptococcus oralis. J. Biomed. Mater. Res. A 2017, 105, 2321–2328. [Google Scholar] [CrossRef]
- Yeniyol, S.; Mutlu, I. Photocatalytical antibacterial activity of mixed-phase TiO2 Nanocomposite Thin Films against Aggregatibacter actinomycetemcomitans. BioMed Res. Int. 2015, 2015. [Google Scholar] [CrossRef]
- Yang, T.; Qian, S. Cytocompatibility and antibacterial activity of titania nanotubes incorporated with gold nanoparticles. Colloids Surf. B Biointerfaces 2016, 145, 597–606. [Google Scholar] [CrossRef]
- Liu, W.; Golshan, N.H. Selenium nanoparticles incorporated into titania nanotubes inhibit bacterial growth and macrophage proliferation. Nanoscale 2016, 8, 15783–15794. [Google Scholar] [CrossRef] [PubMed]
- Besinis, A.; Hadi, S.D. Antibacterial activity and biofilm inhibition by surface modified titanium alloy medical implants following application of silver, titanium dioxide and hydroxyapatite nanocoatings. Nanotoxicology 2017, 11, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Song, Y. Titanium Surface Priming with Phase-Transited Lysozyme to Establish a Silver Nanoparticle-Loaded Chitosan/Hyaluronic Acid Antibacterial Multilayer via Layer-by-Layer Self-Assembly. PLoS ONE 2016, 11, e0146957. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Qiu, J. TiO2 nanorod arrays modified Ti substrates promote the adhesion, proliferation and osteogenic differentiation of human periodontal ligament stem cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 76, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Qiu, J. TiO2 nanorod arrays as a photocatalytic coating enhanced antifungal and antibacterial efficiency of Ti substrates. Nanomedicine (Lond.) 2017, 12, 761–776. [Google Scholar] [CrossRef] [PubMed]
- Yeniyol, S.; He, Z. Antibacterial Activity of As-Annealed TiO2 Nanotubes Doped with Ag Nanoparticles against Periodontal Pathogens. Bioinorg. Chem. Appl. 2014, 2014, 829496. [Google Scholar] [CrossRef]
- Gao, A.; Hang, R. The effects of titania nanotubes with embedded silver oxide nanoparticles on bacteria and osteoblasts. Biomaterials 2014, 35, 4223–4235. [Google Scholar] [CrossRef]
- Wood, N.J.; Jenkinson, H.F. Chlorhexidine hexametaphosphate nanoparticles as a novel antimicrobial coating for dental implants. J. Mater. Sci. Mater. Med. 2015, 26, 201. [Google Scholar] [CrossRef]
- Hang, R.; Gao, A. Antibacterial activity and cytocompatibility of Cu-Ti-O nanotubes. J. Biomed. Mater. Res. A 2014, 102, 1850–1858. [Google Scholar] [CrossRef]
- Toodehzaeim, M.H.; Zandi, H. The Effect of CuO Nanoparticles on Antimicrobial Effects and Shear Bond Strength of Orthodontic Adhesives. J. Dent. (Shiraz) 2018, 19, 1–5. [Google Scholar]
- Wang, X.; Wang, B. Antibacterial orthodontic cement to combat biofilm and white spot lesions. Am. J. Orthod. Dentofac. Orthop. 2015, 148, 974–981. [Google Scholar] [CrossRef]
- Metin-Gursoy, G.; Taner, L. Nanosilver coated orthodontic brackets: In vivo antibacterial properties and ion release. Eur. J. Orthod. 2017, 39, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Sodagar, A.; Akhoundi, M.S.A. Effect of TiO2 nanoparticles incorporation on antibacterial properties and shear bond strength of dental composite used in Orthodontics. Dent. Press J. Orthod. 2017, 22, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Sodagar, A.; Bahador, A. Effect of Addition of Curcumin Nanoparticles on Antimicrobial Property and Shear Bond Strength of Orthodontic Composite to Bovine Enamel. J. Dent. (Tehran) 2016, 13, 373–382. [Google Scholar]
- Sodagar, A.; Akhavan, A. Evaluation of the antibacterial activity of a conventional orthodontic composite containing silver/hydroxyapatite nanoparticles. Prog. Orthod. 2016, 17, 40. [Google Scholar] [CrossRef] [PubMed]
- Degrazia, F.W.; Leitune, V.C. Effect of silver nanoparticles on the physicochemical and antimicrobial properties of an orthodontic adhesive. J. Appl. Oral Sci. 2016, 24, 404–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.J.; Heo, M. Preparation and characterization of antibacterial orthodontic resin containing silver nanoparticles. Appl. Surf. Sci. 2018, 432, 317–323. [Google Scholar] [CrossRef]
- Zaltsman, N.; Kesler Shvero, D. Antibacterial Orthodontic Adhesive Incorporating Polyethyleneimine Nanoparticles. Oral Health Prev. Dent. 2017, 15, 245–250. [Google Scholar] [CrossRef]
- Moreira, D.M.; Oei, J. A novel antimicrobial orthodontic band cement with in situ-generated silver nanoparticles. Angle Orthod. 2015, 85, 175–183. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, N. Novel multifunctional dental cement to prevent enamel demineralization near orthodontic brackets. J. Dent. 2017, 64, 58–67. [Google Scholar] [CrossRef]
- Zhang, N.; Weir, M.D. Orthodontic cement with protein-repellent and antibacterial properties and the release of calcium and phosphate ions. J. Dent. 2016, 50, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Chen, C. Antibacterial and protein-repellent orthodontic cement to combat biofilms and white spot lesions. J. Dent. 2015, 43, 1529–1538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramazanzadeh, B.; Jahanbin, A. Comparison of Antibacterial Effects of ZnO and CuO Nanoparticles Coated Brackets against Streptococcus Mutans. J. Dent. (Shiraz) 2015, 16, 200–205. [Google Scholar]
- Hernandez-Gomora, A.E.; Lara-Carrillo, E. Biosynthesis of Silver Nanoparticles on Orthodontic Elastomeric Modules: Evaluation of Mechanical and Antibacterial Properties. Molecules 2017, 22, 1407. [Google Scholar] [CrossRef] [PubMed]
- Prabha, R.D.; Kandasamy, R. Antibacterial nanosilver coated orthodontic bands with potential implications in dentistry. Indian J. Med. Res. 2016, 144, 580–586. [Google Scholar] [CrossRef]
- Kachoei, M.; Nourian, A. Zinc-oxide nanocoating for improvement of the antibacterial and frictional behavior of nickel-titanium alloy. Nanomed. (Lond.) 2016, 11, 2511–2527. [Google Scholar] [CrossRef]
- Mhaske, A.R.; Shetty, P.C. Antiadherent and antibacterial properties of stainless steel and NiTi orthodontic wires coated with silver against Lactobacillus acidophilus—An in vitro study. Prog. Orthod. 2015, 16, 40. [Google Scholar] [CrossRef]
- Venugopal, A.; Muthuchamy, N. Incorporation of silver nanoparticles on the surface of orthodontic microimplants to achieve antimicrobial properties. Korean J. Orthod. 2017, 47, 3–10. [Google Scholar] [CrossRef]
- Farhadian, N.; Usefi Mashoof, R. Streptococcus mutans counts in patients wearing removable retainers with silver nanoparticles vs those wearing conventional retainers: A randomized clinical trial. Am. J. Orthod. Dentofac. Orthop. 2016, 149, 155–160. [Google Scholar] [CrossRef]
- Mousavi, S.A.; Ghotaslou, R. Antibacterial and antifungal effects of chitosan nanoparticles on tissue conditioners of complete dentures. Int. J. Biol. Macromol. 2018, 118 Pt A, 881–885. [Google Scholar] [CrossRef]
- Yamada, R.; Nozaki, K. Ag nanoparticle-coated zirconia for antibacterial prosthesis. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 78, 1054–1060. [Google Scholar] [CrossRef] [PubMed]
- Suganya, S.; Ahila, S.C. Evaluation and comparison of anti-Candida effect of heat cure polymethylmethacrylate resin enforced with silver nanoparticles and conventional heat cure resins: An in vitro study. Indian J. Dent. Res. 2014, 25, 204–207. [Google Scholar] [CrossRef] [PubMed]
- Jo, J.K.; El-Fiqi, A. Rechargeable microbial anti-adhesive polymethyl methacrylate incorporating silver sulfadiazine-loaded mesoporous silica nanocarriers. Dent. Mater. 2017, 33, e361–e372. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Sun, J. Effect of a denture base acrylic resin containing silver nanoparticles on Candida albicans adhesion and biofilm formation. Gerodontology 2016, 33, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Ginjupalli, K.; Alla, R.K. Antimicrobial activity and properties of irreversible hydrocolloid impression materials incorporated with silver nanoparticles. J. Prosthet. Dent. 2016, 115, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.Y. Characterization and bacterial anti-adherent effect on modified PMMA denture acrylic resin containing platinum nanoparticles. J. Adv. Prosthodont. 2014, 6, 207–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamonkhantikul, K.; Arksornnukit, M. Antifungal, optical, and mechanical properties of polymethylmethacrylate material incorporated with silanized zinc oxide nanoparticles. Int. J. Nanomed. 2017, 12, 2353–2360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alrahlah, A.; Fouad, H. Titanium Oxide (TiO2)/Polymethylmethacrylate (PMMA) Denture Base Nanocomposites: Mechanical, Viscoelastic and Antibacterial Behavior. Materials (Basel) 2018, 11, 1096. [Google Scholar] [CrossRef] [PubMed]
- Totu, E.E.; Nechifor, A.C. Poly(methyl methacrylate) with TiO2 nanoparticles inclusion for stereolitographic complete denture manufacturing—The fututre in dental care for elderly edentulous patients? J. Dent. 2017, 59, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Han, Z. Antibacterial activity, cytotoxicity and mechanical behavior of nano-enhanced denture base resin with different kinds of inorganic antibacterial agents. Dent. Mater. J. 2017, 36, 693–699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gad, M.M.; Al-Thobity, A.M. Inhibitory effect of zirconium oxide nanoparticles on Candida albicans adhesion to repaired polymethyl methacrylate denture bases and interim removable prostheses: A new approach for denture stomatitis prevention. Int. J. Nanomed. 2017, 12, 5409–5419. [Google Scholar] [CrossRef] [PubMed]
- Fathima, J.B.; Pugazhendhi, A. Synthesis and characterization of ZrO2 nanoparticles-antimicrobial activity and their prospective role in dental care. Microb. Pathog. 2017, 110, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Sodagar, A.; Khalil, S. Antimicrobial properties of poly (methyl methacrylate) acrylic resins incorporated with silicon dioxide and titanium dioxide nanoparticles on cariogenic bacteria. J. Orthod. Sci. 2016, 5, 7–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garner, S.J.; Nobbs, A.H. An antifungal coating for dental silicones composed of chlorhexidine nanoparticles. J. Dent. 2015, 43, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jo, J.K. Nano-graphene oxide incorporated into PMMA resin to prevent microbial adhesion. Dent. Mater. 2018, 34, e63–e72. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xie, X. A protein-repellent and antibacterial nanocomposite for Class-V restorations to inhibit periodontitis-related pathogens. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 67, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Melo, M.A. Novel bioactive nanocomposite for Class-V restorations to inhibit periodontitis-related pathogens. Dent. Mater. 2016, 32, e351–e361. [Google Scholar] [CrossRef]
- Wang, L.; Li, C. Novel multifunctional dental bonding agent for Class-V restorations to inhibit periodontal biofilms. Rsc Adv. 2017, 7, 29004–29014. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Xie, X. Effect of bioactive dental adhesive on periodontal and endodontic pathogens. J. Mater. Sci. Mater. Med. 2016, 27. [Google Scholar] [CrossRef]
- Ahrari, F.; Eslami, N. The antimicrobial sensitivity of Streptococcus mutans and Streptococcus sangius to colloidal solutions of different nanoparticles applied as mouthwashes. Dent. Res. J. (Isfahan) 2015, 12, 44–49. [Google Scholar]
- Khan, S.T.; Ahamed, M. Anti-biofilm and antibacterial activities of zinc oxide nanoparticles against the oral opportunistic pathogens Rothia dentocariosa and Rothia mucilaginosa. Eur. J. Oral Sci. 2014, 122, 397–403. [Google Scholar] [CrossRef]
- Mousa, H.M.; Abdal-Hay, A. A Multifunctional Zinc Oxide/Poly(Lactic Acid) Nanocomposite Layer Coated on Magnesium Alloys for Controlled Degradation and Antibacterial Function. ACS Biomater. Sci. Eng. 2018, 4, 2169–2180. [Google Scholar] [CrossRef]
- El-Rashidy, A.A.; Waly, G. Antibacterial activity and biocompatibility of zein scaffolds containing silver-doped bioactive glass. Biomed. Mater. 2018, 13, 065006. [Google Scholar] [CrossRef]
- Besinis, A.; De Peralta, T. Inhibition of biofilm formation and antibacterial properties of a silver nano-coating on human dentin. Nanotoxicology 2014, 8, 745–754. [Google Scholar] [CrossRef]
- Halkai, K.R.; Mudda, J.A. Biosynthesis, Characterization and Antibacterial Efficacy of Silver Nanoparticles Derived from Endophytic Fungi against, P. gingivalis. J. Clin. Diagn. Res. 2017, 11, zc92–zc96. [Google Scholar] [CrossRef]
- Mendes-Gouvea, C.C.; do Amaral, J.G. Sodium trimetaphosphate and hexametaphosphate impregnated with silver nanoparticles: Characteristics and antimicrobial efficacy. Biofouling 2018, 34, 299–308. [Google Scholar] [CrossRef]
- Panacek, A.; Smekalova, M. Silver nanoparticles strongly enhance and restore bactericidal activity of inactive antibiotics against multiresistant Enterobacteriaceae. Colloids Surf B Biointerfaces 2016, 142, 392–399. [Google Scholar] [CrossRef]
- Niska, K.; Knap, N. Capping Agent-Dependent Toxicity and Antimicrobial Activity of Silver Nanoparticles: An In Vitro Study. Concerns about Potential Application in Dental Practice. Int. J. Med. Sci. 2016, 13, 772–782. [Google Scholar] [CrossRef] [Green Version]
- Scarpelli, B.B.; Punhagui, M.F. In Vitro Evaluation of the Remineralizing Potential and Antimicrobial Activity of a Cariostatic Agent with Silver Nanoparticles. Braz. Dent. J. 2017, 28, 738–743. [Google Scholar] [CrossRef]
- Panpaliya, N.P.; Dahake, P.T. In vitro evaluation of antimicrobial property of silver nanoparticles and chlorhexidine against five different oral pathogenic bacteria. Saudi Dent. J. 2018. [Google Scholar] [CrossRef]
- Priyadarshini, B.M.; Fawzy, A.S. Potentiating the antibacterial effect of silver nanospheres by surface-capping with chlorhexidine gluconate. J. Nanopart. Res. 2017, 19. [Google Scholar] [CrossRef]
- Sanchez, M.C.; Toledano-Osorio, M. Antibacterial effects of polymeric PolymP-n Active nanoparticles. An. in vitro biofilm study. Dent. Mater. 2018. [Google Scholar] [CrossRef] [PubMed]
- Kivanc, M.; Barutca, B. Effects of hexagonal boron nitride nanoparticles on antimicrobial and antibiofilm activities, cell viability. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 91, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Soekanto, S.A.; Marpaung, L.J. Efficacy of propolis fluoride and nano silver fluoride for inhibition of streptococcus mutans and enterococcus faecalis biofilm formation. Int. J. Appl. Pharm. 2017, 9, 51–54. [Google Scholar] [CrossRef]
- Ribeiro Targino, A.G.; Pelagio Flores, M.A. An innovative approach to treating dental decay in children. A new anti-caries agent. J. Mater. Sci. Mater. Med. 2014, 25, 2041–2047. [Google Scholar] [CrossRef] [PubMed]
- Freire, P.L.L.; Albuquerque, A.J.R. AgNPs: The New Allies against S. Mutans Biofilm—A Pilot Clinical Trial and Microbiological Assay. Braz. Dent. J. 2017, 28, 417–422. [Google Scholar] [CrossRef]
- Mackevica, A.; Olsson, M.E. The release of silver nanoparticles from commercial toothbrushes. J. Hazard. Mater. 2017, 322, 270–275. [Google Scholar] [CrossRef] [Green Version]
- Do Nascimento, C.; Paulo, D.F. Microbial diversity of the supra- and subgingival biofilm of healthy individuals after brushing with chlorhexidine- or silver-coated toothbrush bristles. Can. J. Microbiol. 2014, 61, 112–123. [Google Scholar] [CrossRef]
- Ikono, R.; Mardliyati, E. Chitosan-PRP nanosphere as a growth factors slow releasing device with superior antibacterial capability. Biomed. Phys. Eng. Express 2018, 4. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim da, E. pH-Responsive mineralized nanoparticles as stable nanocarriers for intracellular nitric oxide delivery. Colloids Surf. B Biointerfaces 2016, 146, 1–8. [Google Scholar] [CrossRef]
- Rencber, S.; Karavana, S.Y. Development, characterization, and in vivo assessment of mucoadhesive nanoparticles containing fluconazole for the local treatment of oral candidiasis. Int. J. Nanomed. 2016, 11, 2641–2653. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, W.; Ge, S. Application of Antimicrobial Nanoparticles in Dentistry. Molecules 2019, 24, 1033. https://doi.org/10.3390/molecules24061033
Song W, Ge S. Application of Antimicrobial Nanoparticles in Dentistry. Molecules. 2019; 24(6):1033. https://doi.org/10.3390/molecules24061033
Chicago/Turabian StyleSong, Wenjing, and Shaohua Ge. 2019. "Application of Antimicrobial Nanoparticles in Dentistry" Molecules 24, no. 6: 1033. https://doi.org/10.3390/molecules24061033
APA StyleSong, W., & Ge, S. (2019). Application of Antimicrobial Nanoparticles in Dentistry. Molecules, 24(6), 1033. https://doi.org/10.3390/molecules24061033