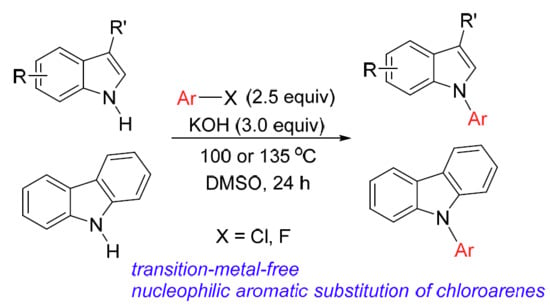
Base-Promoted SNAr Reactions of Fluoro- and Chloroarenes as a Route to N-Aryl Indoles and Carbazoles
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Methods
3.2. Typical Experiment Procedure for the Synthesis of 3aa
3.3. Typical Experiment Procedure for the Synthesis of 5a
3.4. Characterization Data of Products
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References and Notes
- Hartwig, J.F. Evolution of a Fourth Generation Catalyst for the Amination and Thioetherification of Aryl Halides. Acc. Chem. Res. 2008, 41, 1534–1544. [Google Scholar] [CrossRef] [Green Version]
- Surry, D.S.; Buchwald, S.L. Dialkylbiaryl Phosphines in Pd-catalyzed Amination: A User’s Guide. Chem. Sci. 2011, 2, 27–50. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Castillo, P.; Buchwald, S.L. Applications of Palladium-Catalyzed C–N Cross-Coupling Reactions. Chem. Rev. 2016, 116, 12564–12649. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.-H.; Lin, P.-S.; Lee, Y.-M.; Shen, W.-T.; Wu, M.-J. Palladium(II)-Catalyzed One-Pot Syntheses of 9-(Pyridin-2-yl)-9H-carbazoles through a Tandem C-H Activation/C-X (X = C or N) Formation Process. Chem. Eur. J. 2011, 17, 13613–13620. [Google Scholar] [CrossRef] [PubMed]
- Maejima, T.; Shimoda, Y.; Nozaki, K.; Mori, S.; Sawama, Y.; Monguchi, Y.; Sajiki, H. One-pot Aromatic Amination based on Carbon–Nitrogen Coupling Reaction Between Aryl Halides and Azido Compounds. Tetrahedron 2012, 68, 1712–1722. [Google Scholar] [CrossRef]
- Ghorai, S.K.; Gopalsamuthiram, V.G.; Jawalekar, A.M.; Patre, R.E.; Pal, S. Iron Catalyzed C-N Bond Formation. Tetrahedron 2017, 73, 1769–1794. [Google Scholar] [CrossRef]
- Trump, R.P.; Blanc, J.-B.E.; Stewart, E.L.; Brown, P.J.; Caivano, M.; Gray, D.W.; Hoekstra, W.J.; Willson, T.M.; Han, B.; Turnbull, P. Design and Synthesis of an Array of Selective Androgen Receptor Modulators. J. Comb. Chem. 2007, 9, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, H.; Xing, X.; Xu, Y.; Ma, J.-A.; Zhang, B. Selective C4–F Bond Cleavage of Pentafluorobenzene: Synthesis of N-Tetrafluoroarylated Heterocyclic Compounds. Tetrahedron Lett. 2013, 54, 4649–4652. [Google Scholar] [CrossRef]
- Diness, F.; Fairlie, D.P. Catalyst-free N-arylation using unactivated fluorobenzenes. Angew. Chem. Int. Ed. 2012, 51, 8012–8016. [Google Scholar] [CrossRef]
- Podolan, G.; Jungk, P.; Lentz, D.; Zimmer, R.; Reissig, H.-U. Studies on the Synthesis of Specifically Fluorinated 4-Amino-pyridine Derivatives by Regioselective Nucleophilic Aromatic Substitution and Catalytic Hydrodefluorination. Adv. Synth. Catal. 2015, 357, 3215–3228. [Google Scholar] [CrossRef]
- Yang, S.; Wu, C.; Ruan, M.; Yang, Y.; Zhao, Y.; Niu, J.; Yang, W.; Xu, J. Metal- and Ligand-Free Ullmann-Type C–O and C–N Coupling Reactions Promoted by Potassium t-Butoxide. Tetrahedron Lett. 2012, 53, 4288–4292. [Google Scholar] [CrossRef]
- Schmidt, E.Y.; Ivanova, E.V.; Tatarinova, I.V.; Ushakov, I.A.; Semenova, N.V.; Vashchenko, A.V.; Trofimov, B.A. Synthesis of Acyl Terphenyls and Higher Polyaromatics via Base-Promoted C–H Functionalization of Acetylarenes with Arylacetylenes. Org. Lett. 2016, 18, 2158–2161. [Google Scholar] [CrossRef] [PubMed]
- Vitkovskaya, N.M.; Kobychev, V.B.; Skitnevskaya, A.D.; Orel, V.B.; Bobkov, A.S.; Zubarev, A.A.; Trofimov, B.A. Synthesis of Divinyl Sulfide via Addition of the Hydrogen Sulfide Anion to Acetylene in an Alkaline Metal Hydroxide/DMSO Superbasic System: A Quantum-Chemical Insight. Tetrahedron Lett. 2017, 58, 92–96. [Google Scholar] [CrossRef]
- Petrova, O.V.; Sobenina, L.N.; Budaev, A.B.; Ivanov, A.V.; Samsonov, V.A.; Tikhonov, A.Y.; Trofimov, B.A. Formation of 1-Aminophenazine from 3,4-Dihydrophenazin-1(2H)-one Oxime in the System Acetylene–KOH–DMSO. Russ. J. Org. Chem. 2017, 53, 150–152. [Google Scholar] [CrossRef]
- Schmidt, E.Y.; Bidusenko, I.A.; Ushakov, I.A.; Vashchenko, A.V.; Trofimov, B.A. Decorated Cyclopentadienes from Acetylene and Ketones in Just Two Steps. Org. Lett. 2017, 19, 3127–3130. [Google Scholar] [CrossRef]
- Trofimov, B.A.; Schmidt, E.Y. Acetylenes in the Superbase-Promoted Assembly of Carbocycles and Heterocycles. Acc. Chem. Res. 2018, 51, 1117–1130. [Google Scholar] [CrossRef]
- Orel, V.B.; Vitkovskaya, N.M.; Kobychev, V.B.; Trofimov, B.A. Transition-Metal-Free C-Vinylation of Ketones with Acetylenes: A Quantum-Chemical Rationalization of Similarities and Differences in Catalysis by Superbases MOH/DMSO and tBuOM/DMSO (M = Na, K). J. Org. Chem. 2018, 83, 3719–3726. [Google Scholar] [CrossRef]
- Yuan, Y.; Thomé, I.; Kim, S.H.; Chen, D.; Beyer, A.; Bonnamour, J.; Zuidema, E.; Chang, S.; Bolm, C. Dimethyl Sulfoxide/Potassium Hydroxide: A Superbase for the Transition Metal-Free Preparation of Cross-Coupling Products. Adv. Synth. Catal. 2010, 352, 2892–2898. [Google Scholar] [CrossRef]
- Beyer, A.; Reucher, C.M.M.; Bolm, C. Potassium Hydroxide/Dimethyl Sulfoxide Promoted Intramolecular Cyclization for the Synthesis of Benzimidazol-2-ones. Org. Lett. 2011, 13, 2876–2879. [Google Scholar] [CrossRef]
- Baars, H.; Beyer, A.; Kohlhepp, S.V.; Bolm, C. Transition-Metal-Free Synthesis of Benzimidazoles Mediated by KOH/DMSO. Org. Lett. 2014, 16, 536–539. [Google Scholar] [CrossRef]
- Hendriks, C.M.M.; Bohmann, R.A.; Bohlem, M.; Bolm, C. N-Alkylations of NH-Sulfoximines and NH-Sulfondiimines with Alkyl Halides Mediated by Potassium Hydroxide in Dimethyl Sulfoxide. Adv. Synth. Catal. 2014, 356, 1847–1852. [Google Scholar] [CrossRef]
- Xie, S.; Zhang, Y.; Ramstrom, O.; Yan, M. Base-Catalyzed Synthesis of Aryl Amides from Aryl Azides and Aldehydes. Chem. Sci. 2016, 7, 713–718. [Google Scholar] [CrossRef]
- Gao, L.; Chang, B.; Qiu, W.; Wang, L.; Fu, X.; Yuan, R. Potassium Hydroxide/Dimethyl Sulfoxide Superbase-Promoted Transition Metal-Free Synthesis of 2-Substituted Benzothiophenes under Visible Light. Adv. Synth. Catal. 2016, 358, 1202–1207. [Google Scholar] [CrossRef]
- Rehan, M.; Maity, S.; Morya, L.K.; Pal, K.; Ghorai, P. Transition-Metal-Free Synthesis of Homo- and Hetero-1,2,4-Triaryl Benzenes by an Unexpected Base-Promoted Dearylative Pathway. Angew. Chem. Int. Ed. 2016, 55, 7728–7732. [Google Scholar] [CrossRef]
- Zheng, Q.; Hua, R.; Jiang, J.; Zhang, L. A General Approach to Arylated Furans, Pyrroles, and Thiophenes. Tetrahedron 2014, 70, 8252–8256. [Google Scholar] [CrossRef]
- Su, J.; Chen, Q.; Lu, L.; Ma, Y.; Auyoung, G.H.L.; Hua, R. Base-Promoted Nucleophilic Fluoroarenes Substitution of C-F Bonds. Tetrahedron 2018, 74, 303–307. [Google Scholar] [CrossRef]
- Yi, C.; Hua, R. Efficient Copper-Free PdCl2(PCy3)2-Catalyzed Sonogashira Coupling of Aryl Chlorides with Terminal Alkynes. J. Org. Chem. 2006, 71, 2535–2537. [Google Scholar] [CrossRef]
- Yi, C.; Hua, R. An Efficient Palladium-Catalyzed Heck Coupling of Aryl Chlorides with Alkenes. Tetrahedron Lett. 2006, 47, 2573–2576. [Google Scholar] [CrossRef]
- Yi, C.; Hua, R. Palladium-Catalyzed Efficient and One-Pot Synthesis of Diarylacetylenes from the Reaction of Aryl Chlorides with 2-Methyl-3-butyn-2-ol. Adv. Synth. Catal. 2007, 349, 1738–1742. [Google Scholar] [CrossRef]
- Li, M.; Hua, R. Palladium-Catalyzed Heck Voupling of 2-Vinylpyridine with Aryl Chlorides. Appl. Organomet. Chem. 2008, 22, 397–401. [Google Scholar] [CrossRef]
- Qi, C.; Zheng, Q.; Hua, R. A Domino Three-Component Condensation of ortho-Haloacetophenones with Urea or Amines: A Novel One-Pot Synthesis of Halogen-Substituted Quinolones. Tetrahedron 2009, 65, 1316–1320. [Google Scholar] [CrossRef]
- Ju, J.; Qi, C.; Zheng, L.; Hua, R. Synthesis of 3-Methyleneisoindolin-1-ones via Palladium-Catalyzed C–Cl Bond Cleavage and Cyclocarbonylation of ortho-Chloro Ketimines. Tetrahedron Lett. 2013, 54, 5159–5161. [Google Scholar] [CrossRef]
- Old, D.W.; Harris, M.C.; Buchwald, S.L. Efficient Palladium-Catalyzed N-Arylation of Indoles. Org. Lett. 2000, 2, 1403–1406. [Google Scholar] [CrossRef]
- Talukdar, D.; Das, G.; Thakur, S.; Karak, N.; Thakur, A.J. Copper Nanoparticle Decorated Organically Modified Montmorillonite (OMMT): An Efficient Catalyst for the N-Arylation of Indoles and Similar Heterocycles. Catal. Commun. 2015, 59, 238–243. [Google Scholar] [CrossRef]
- Modha, S.G.; Greaney, M.F. Atom-Economical Transformation of Diaryliodonium Salts: Tandem C–H and N–H Arylation of Indoles. J. Am. Chem. Soc. 2015, 137, 1416–1419. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; She, Y.; Fang, K.; Li, G. CuCl-Catalyzed Ullmann-Type C–N Cross-Coupling Reaction of Carbazoles and 2-Bromopyridine Derivatives. J. Org. Chem. 2017, 82, 1024–1033. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Liu, N.; Ji, E.; Dai, B. Copper/β-Diketone-Catalysed N-Arylation of Carbazoles. RSC Adv. 2015, 5, 51512–51523. [Google Scholar] [CrossRef]
- Recently, the C-N coupling of indoles and carbazoles with aromatic chlorides catalyzed by NHC-Nickel (0) precursor has been reported, see: Rull, S.G.; Blandez, J.F.; Fructos, M.R.; Belderrain, T.R.; Nicasio, M.C. C-N Coupling of Indoles and Carbazoles with Aromatic Chlorides Catalyzed by a Single-Component NHC-Nickel(0) Precursor. Adv. Synth. Catal. 2015, 357, 907–911. [Google Scholar] [CrossRef]
- Xu, H.; Fan, L.-L. Microwave-assisted N-arylation of indoles via C(sp2)–N(sp2) bond formation by aromatic nucleophilic substitution reactions. Z. Naturforsch. 2008, 63b, 298–302. [Google Scholar] [CrossRef]
- Guo, F.; Wang, L.; Wang, P.; Yu, J.; Han, J. Transition-metal-free N-arylation of carbazoles and C-arylation of tetrahydrocarbazoles by using diaryliodonium salts. Asian J. Org. Chem. 2012, 1, 218–221. [Google Scholar] [CrossRef]
- The structure of 3aq was confirmed by X-ray crystallography (see supplementary materials). The supplementary crystallographic data, CCDC 1903659 can be obtained free of charge via www.ccdc.cam.ac.uk.
- Wang, C.; Dong, H.; Hu, W.; Liu, Y.; Zhu, D. Semiconducting π-Conjugated Systems in Field-Effect Transistors: A Material Odyssey of Organic Electronics. Chem. Rev. 2012, 112, 2208–2267. [Google Scholar] [CrossRef] [PubMed]
- Wex, B.; Kaafarani, B.R. Perspective on Carbazole-Based Organic Compounds as Emitters and Hosts in TADF Applications. J. Mater. Chem. C 2017, 5, 8622–8653. [Google Scholar] [CrossRef]
- Wei, J.J.; Song, W.B.; Zhu, Y.F.; Wei, B.L.; Xuan, L.J. N,N-Dimethyl-d-glucosamine as an Efficient Ligand for Copper-catalyzed Ullmann-type Coupling of N-H Heterocycles with Aryl Halides. Tetrahedron 2018, 74, 19–27. [Google Scholar] [CrossRef]
- Veisi, H.; Morakabati, N. Palladium Nanoparticles Supported on Modified Single-Walled Carbon Nanotubes: A Heterogeneous and Reusable Catalyst in the Ullmann-type N-Arylation of Imidazoles and Indoles. New J. Chem. 2015, 39, 2901–2907. [Google Scholar] [CrossRef]
- Wu, H.; Liu, T.; Cui, M.; Li, Y.; Jian, J.; Wang, H.; Zeng, Z. Rhodium-catalyzed C–H functionalization with N-acylsaccharins. Org. Biomol. Chem. 2017, 15, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Takahashi, Y.; Matsubara, Y.; Yamada, K. An Ullmann N-Arylation/2-Amidation Cascade by Self-relay Copper Catalysis: One-pot Synthesis of Indolo[1,2-a]quinazolinones. Org. Chem. Front. 2017, 4, 2124–2127. [Google Scholar] [CrossRef]
- Verma, A.K.; Singh, J.; Larock, R.C. Benzotriazole: An Efficient Ligand for the Copper-Catalyzed N-Arylation of Indoles. Tetrahedron 2009, 65, 8434–8439. [Google Scholar] [CrossRef]
- Yong, F.-F.; Teo, Y.-C.; Tay, S.-H.; Tan, B.Y.-H.; Lim, K.-H. A Ligand-Free Copper(I) Oxide Catalyzed Strategy for the N-Arylation of Azoles in Water. Tetrahedron Lett. 2011, 52, 1161–1164. [Google Scholar] [CrossRef]
- Chang, D.; Gao, F.; Shi, L. Potassium t-Butoxide-mediated Generation of Arynes from o-Bromoacetophenone Derivatives. Tetrahedron 2018, 74, 2428–2434. [Google Scholar] [CrossRef]
- Kancherla, R.; Naveen, T.; Maiti, D. Divergent Reactivity in Palladium-Catalyzed Annulation with Diarylamines and α,β-Unsaturated Acids: Direct Access to Substituted 2-Quinolinones and Indoles. Chem. Eur. J. 2015, 21, 8723–8726. [Google Scholar] [CrossRef]
- Mangione, M.I.; Spanevello, R.A.; Anzardi, M.B. Efficient and Straightforward Click Synthesis of Structurally Related Dendritic Triazoles. RSC Adv. 2017, 7, 47681–47688. [Google Scholar] [CrossRef]
- Leitch, J.A.; Heron, C.J.; McKnight, J.; Kociok-Köhn, G.; Bhonoah, Y.; Frost, C.G. Ruthenium Catalyzed Remote C4-selective C–H Functionalisation of Carbazoles via σ-Activation. Chem. Commun. 2017, 53, 13039–13042. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the products are available from the authors. |



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iqbal, M.A.; Mehmood, H.; Lv, J.; Hua, R. Base-Promoted SNAr Reactions of Fluoro- and Chloroarenes as a Route to N-Aryl Indoles and Carbazoles. Molecules 2019, 24, 1145. https://doi.org/10.3390/molecules24061145
Iqbal MA, Mehmood H, Lv J, Hua R. Base-Promoted SNAr Reactions of Fluoro- and Chloroarenes as a Route to N-Aryl Indoles and Carbazoles. Molecules. 2019; 24(6):1145. https://doi.org/10.3390/molecules24061145
Chicago/Turabian StyleIqbal, Muhammad Asif, Hina Mehmood, Jiaying Lv, and Ruimao Hua. 2019. "Base-Promoted SNAr Reactions of Fluoro- and Chloroarenes as a Route to N-Aryl Indoles and Carbazoles" Molecules 24, no. 6: 1145. https://doi.org/10.3390/molecules24061145
APA StyleIqbal, M. A., Mehmood, H., Lv, J., & Hua, R. (2019). Base-Promoted SNAr Reactions of Fluoro- and Chloroarenes as a Route to N-Aryl Indoles and Carbazoles. Molecules, 24(6), 1145. https://doi.org/10.3390/molecules24061145