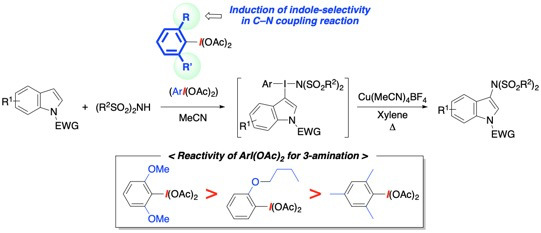
3.2. General Procedure for 3-Amination of Indoles 1
A mixture of 1-(diacetoxyiodo)-2-butoxybenzene (94.6 mg, 0.24 mmol) and Ts2NH (104.1 mg, 0.32 mmol) in MeCN (2.0 mL) was stirred at room temperature for 30 min under argon atmosphere. Then, N-pivaloylindole (1a) (40.3 mg, 0.20 mmol) was added, and the solution was stirred at 40 °C for 7 h under argon atmosphere. The reaction mixture was concentrated under reduced pressure and the crude product was dissolved xylene (mixed isomers) (2.0 mL). Cu(MeCN)4BF4 (3.1 mg, 0.01 mmol) was added to the reaction mixture at room temperature, and further stirred at 130 °C for 1 h under argon atmosphere. Saturated NaHCO3 aqueous solution (10 mL) was added to the reaction mixture, and the product was extracted with AcOEt (15 mL × 3). The combined extracts were washed with brine (10 mL) and dried over Na2SO4. The organic phase was concentrated under reduced pressure and the crude product was purified by silica gel column chromatography (eluent: hexane/AcOEt = 5/1), to give the desired product 2a (82.3 mg, 78% yield).
4-Methyl-N-(1-pivaloyl-1H-indol-3-yl)-N-tosylbenzenesulfonamide (
2a). White solid, mp 214.0–214.5 °C,
1H NMR (500 MHz, CDCl
3) δ 1.40 (s, 9H), 2.46 (s, 6H), 7.07 (d,
J = 7.8 Hz, 1H), 7.14–7.19 (m, 1H), 7.30–7.37 (m, 1H), 7.32 (d,
J = 8.2 Hz, 4H), 7.46 (s, 1H), 7.87 (d,
J = 8.2 Hz, 4H), 8.46 (d,
J = 8.3 Hz, 1H).
13C NMR (125 MHz, CDCl
3) δ 21.7 (2C), 28.5 (3C), 41.3, 115.8, 117.3, 118.6, 124.1, 126.0, 126.8, 127.7, 128.6 (4C), 129.6 (4C), 135.7, 136.2 (2C), 145.2 (2C), 176.7. IR (neat) 1702, 1374, 1357, 1316, 1165 cm
–1. MS (ESI) calcd for C
27H
29N
2O
5S
2 [M + H]
+ 525.1512, found 525.1501. Crystal data for 2a: Formula C
27H
28N
2O
5S
2, colorless, crystal dimensions 0.30 × 0.20 × 0.20 mm
3, Triclinic, space group P −1,
a = 9.475(2) Å,
b = 10.144(2) Å,
c = 13.660(3) Å, α = 98.118(3) °, β = 91.348(3) °, γ = 100.871(3) °,
V = 1274.7(5) Å
3,
Z = 2, ρ
calc = 1.367 g cm
−3, F(000) = 552, μ(MoKα) = 0.250 mm
−1,
T = 173 K. 7413 reflections collected, 5602 independent reflections with
I > 2σ(
I) (2θ
max = 27.572°), and 330 parameters were used for the solution of the structure. The non-hydrogen atoms were refined anisotropically.
R1 = 0.0508 and
wR2 = 0.1031. GOF = 1.015. Crystallographic data (excluding structure factors) for the structure reported in this paper have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication no. CCDC-1860598. Copies of the data can be obtained free of charge on application to CCDC, 12 Union Road, Cambridge CB2 1EZ, UK [Fax: int. code + 44(1223)336-033; E-mail:
[email protected]].
N-(5-Chloro-1-pivaloyl-1H-indol-3-yl)-4-methyl-N-tosylbenzenesulfonamide (2b): White solid, mp 205.5–206.0 °C, 1H NMR (500 MHz, CDCl3) δ 1.41 (s, 9H), 2.48 (s, 6H), 6.81 (d, J = 2.0 Hz, 1H), 7.27 (d, J = 8.9, 2.0 Hz, 1H), 7.33 (d, J = 8.5 Hz, 4H), 7.52 (s, 1H), 7.85 (d, J = 8.5 Hz, 4H), 8.38 (d, J = 8.9 Hz, 1H). 13C NMR (125 MHz, CDCl3) δ 21.7 (2C), 28.4 (3C), 41.3, 115.2, 118.2, 118.4, 126.2, 128.0, 128.6 (4C), 128.7, 129.7 (4C), 130.0, 133.9, 136.0 (2C), 145.6 (2C), 176.6. IR (neat) 1708, 1379, 1351, 1307, 1163, 1085 cm–1. MS (ESI) calcd for C27H28ClN2O5S2 [M + H]+ 559.1123, found 559.1119.
N-(Phenylsulfonyl)-N-(1-pivaloyl-1H-indol-3-yl)benzenesulfonamide (2c). White solid, mp 182.0–183.0 °C, 1H NMR (500 MHz, CDCl3) δ 1.39 (s, 9H), 7.01 (d, J = 8.0 Hz, 1H), 7.12–7.18 (m, 1H), 7.31–7.37 (m, 1H), 7.48 (s, 1H), 7.50–7.56 (m, 4H), 7.67 (t, J = 7.5 Hz, 2H), 7.97–8.02 (m, 4H), 8.47 (d, J = 8.5 Hz, 1H). 13C NMR (125 MHz, CDCl3) δ 28.5 (3C), 41.3, 115.5, 117.3, 118.5, 124.2, 126.1, 126.6, 127.7, 128.5 (4C), 129.0 (4C), 134.1 (2C), 135.6, 139.0 (2C), 176.7. IR (neat) 1704, 1381, 1344, 1315, 1159 cm–1. MS (ESI) calcd for C25H25N2O5S2 [M + H]+ 497.1199, found 497.1205.
4-Methyl-N-(methylsulfonyl)-N-(1-pivaloyl-1H-indol-3-yl)benzenesulfonamide (2d). White solid, mp 221.5–22.0 °C, 1H NMR (500 MHz, CDCl3) δ 1.44 (s, 9H), 2.43 (s, 3H), 3.56 (s, 3H), 7.23–7.30 (m, 3H), 7.30–7.34 (m, 1H), 7.34–7.40 (m, 1H), 7.62 (s, 1H), 7.79 (d, J = 8.3 Hz, 2H), 8.48 (d, J = 8.3 Hz, 1H). 13C NMR (125 MHz, CDCl3) δ 21.7, 28.5 (3C), 41.4, 44.0, 115.2, 117.4, 118.2, 124.4, 126.2, 126.7, 127.2, 128.8 (2C), 129.6 (2C), 135.1, 135.7, 145.6, 176.7. IR (neat) 1704, 1366, 1349, 1317, 1157 cm–1. MS (ESI) calcd for C21H25N2O5S2 [M + H]+ 449.1199, found 449.1198.
N-(1-Pivaloyl-1H-indol-3-yl)-N-(propylsulfonyl)propane-1-sulfonamide (2e). White solid, mp 190.0–191.0 °C, 1H NMR (500 MHz, CDCl3, 60 °C) δ 1.07 (t, J = 7.3 Hz, 6H), 1.52 (s, 9H), 1.88–2.01 (m, 4H), 3.37–3.55 (m, 2H), 3.55–3.75 (m, 2H), 7.31–7.41 (m, 2H), 7.62 (d, J = 7.5 Hz, 1H), 7.88 (s, 1H), 8.50 (d, J = 8.0 Hz, 1H). 13C NMR (125 MHz, CDCl3, 60 °C) δ 12.8 (2C), 17.1 (2C), 28.7 (3C), 41.5, 57.7 (2C), 115.5, 117.6, 118.5, 124.5, 126.2, 126.9, 127.2, 135.9, 176.7. IR (neat) 1703, 1370, 1347, 1316, 1151 cm–1. MS (ESI) calcd for C19H29N2O5S2 [M + H]+ 429.1512, found 429.1512.
N-(Methylsulfonyl)-N-(1-pivaloyl-1H-indol-3-yl)methanesulfonamide (2f). White solid, mp 205.0–207.0 °C, 1H NMR (500 MHz, CDCl3) δ 1.54 (s, 9H), 3.44 (s, 6H), 7.35–7.40 (m, 1H), 7.40–7.45 (m, 1H), 7.58–7.63 (m, 1H), 7.94 (s, 1H), 8.53 (d, J = 8.3 Hz, 1H). 13C NMR (125 MHz, CDCl3) δ 28.6 (3C), 41.5, 42.9 (2C), 114.6, 117.7, 118.0, 124.7, 126.3, 126.4, 127.1, 135.8, 176.8. IR (neat) 1703, 1349, 1317, 1159 cm–1. MS (ESI) calcd for C15H20ClN2O5S2 [M + Cl]− 407.0508, found 407.0515.
4-Methyl-N-(5-methyl-1-pivaloyl-1H-indol-3-yl)-N-tosylbenzenesulfonamide (2g). White solid, mp 217.0–217.5 °C, 1H NMR (500 MHz, CDCl3) δ 1.39 (s, 9H), 2.28 (s, 3H), 2.47 (s, 6H), 6.71–6.73 (m, 1H), 7.12–7.16 (m, 1H), 7.32 (d, J = 8.2 Hz, 4H), 7.42 (s, 1H), 8.87 (d, J = 8.2 Hz, 4H), 8.31 (d, J = 8.6 Hz, 1H). 13C NMR (125 MHz, CDCl3) δ 21.2, 21.7 (2C), 28.5 (3C), 41.2, 115.6, 116.9, 118.4, 126.9, 127.4, 127.6, 128.7 (4C), 129.5 (4C), 133.8, 133.9, 136.3 (2C), 145.2 (2C), 176.6. IR (neat) 1701, 1379, 1353, 1308, 1156 cm–1. MS (ESI) calcd for C28H31N2O5S2 [M + H]+ 539.1669, found 539.1672.
N-(5-Fluoro-1-pivaloyl-1H-indol-3-yl)-4-methyl-N-tosylbenzenesulfonamide (2h). White solid, mp 214.5–215.2 °C, 1H NMR (500 MHz, CDCl3) δ 1.40 (s, 9H), 2.47 (s, 6H), 6.66 (dd, J = 8.5, 2.5 Hz, 1H), 7.05 (td, J = 9.0, 2.5 Hz, 1H), 7.33 (d, J = 8.5 Hz, 4H), 7.51 (s, 1H), 7.86 (d, J = 8.5 Hz, 4H), 8.43 (dd, J = 9.0, 4.5 Hz, 1H). 13C NMR (125 MHz, CDCl3) δ 21.7 (2C), 28.5 (3C), 41.3, 104.3 (d, JC–F = 25.0 Hz), 113.9 (d, JC–F = 25.0 Hz), 115.5 (d, JC–F = 3.5 Hz), 118.6 (d, JC–F = 9.5 Hz), 128.1 (d, JC–F = 9.5 Hz), 128.5 (4C), 129.0, 129.7 (4C), 131.9, 136.1 (2C), 145.5 (2C), 159.9 (d, JC–F = 240.8 Hz), 176.5. 19F NMR (471 MHz, CDCl3) δ −117.9. IR (neat) 1708, 1376, 1359, 1311, 1237, 1169 cm–1. MS (ESI) calcd for C27H28FN2O5S2 [M + H]+ 543.1418, found 543.1422.
N-(5-Bromo-1-pivaloyl-1H-indol-3-yl)-4-methyl-N-tosylbenzenesulfonamide (2i). White solid, mp 215.0–216.0 °C, 1H NMR (500 MHz, CDCl3) δ 1.41 (s, 9H), 2.48 (s, 6H), 6.09 (d, J = 1.8 Hz, 1H), 7.31–7.35 (m, 4H), 7.40 (dd, J = 9.0, 1.8 Hz, 1H), 7.51 (s, 1H), 7.82–7.86 (m, 4H), 8.32 (d, J = 9.0 Hz, 1H). 13C NMR (125 MHz, CDCl3) δ 21.7 (2C), 28.4 (3C), 41.3, 115.0, 117.7, 118.7, 121.3, 128.4, 128.50, 128.55 (4C), 128.8, 129.7 (4C), 134.3, 136.0 (2C), 145.6 (2C), 176.6. IR (neat) 1703, 1382, 1348, 1306, 1164, 659 cm–1. MS (ESI) calcd for C27H28BrN2O5S2 [M + H]+ 603.0618, found 603.0620.
N-(5-Methoxy-1-pivaloyl-1H-indol-3-yl)-4-methyl-N-tosylbenzenesulfonamide (2j). White solid, mp 220.0–221.0 °C, 1H NMR (500 MHz, CDCl3) δ 1.39 (s, 9H), 2.46 (s, 6H), 3.62 (s, 3H), 6.37 (d, J = 2.6 Hz, 1H), 6.92 (dd, J = 9.1, 2.6 Hz, 1H), 7.32 (d, J = 8.2 Hz, 4H), 7.43 (s, 1H), 7.88 (d, J = 8.2 Hz, 4H), 8.34 (d, J = 9.1 Hz, 1H). 13C NMR (125 MHz, CDCl3) δ 21.7 (2C), 28.6 (3C), 41.2, 55.2, 100.1, 115.4, 115.6, 118.3, 127.8, 128.0, 128.6 (4C), 129.6 (4C), 130.2, 136.4 (2C), 145.2 (2C), 156.7, 176.4. IR (neat) 1701, 1380, 1358, 1312, 1162, 1086 cm–1. MS (ESI) calcd for C28H31N2O6S2 [M + H]+ 555.1618, found 555.1621.
3-((4-Methyl-N-tosylphenyl)sulfonamido)-1-pivaloyl-1H-indol-5-yl pivalate (2k): White solid, mp 225.0 °C (dec.), 1H NMR (500 MHz, CDCl3) δ 1.35 (s, 9H), 1.40 (s, 9H), 2.45 (s, 6H), 6.64 (d, J = 2.5 Hz, 1H), 7.02 (dd, J = 9.0, 2.5 Hz, 1H), 7.33 (d, J = 8.3 Hz, 4H), 7.48 (s, 1H), 7.88 (d, J = 8.3 Hz, 4H), 8.46 (d, J = 9.0 Hz, 1H). 13C NMR (125 MHz, CDCl3) δ 21.7 (2C), 27.1 (3C), 28.5 (3C), 38.9, 41.3, 113.3, 115.7, 118.1, 120.0, 127.6, 128.58 (4C), 128.63, 129.7 (4C), 133.2, 136.2 (2C), 145.3 (2C), 147.7, 176.5, 176.8. IR (neat) 1750, 1708, 1377, 1358, 1311, 1167, 1119 cm–1. MS (ESI) calcd for C32H37N2O7S2 [M + H]+ 625.2037, found 625.2937.
Methyl 3-((4-methyl-N-tosylphenyl)sulfonamido)-1-pivaloyl-1H-indole-5-carboxylate (2l). White solid, mp 221.0–221.5 °C, 1H NMR (500 MHz, CDCl3) δ 1.41 (s, 9H), 2.46 (s, 6H), 3.89 (s, 3H), 7.33 (d, J = 8.3 Hz, 4H), 7.54 (s, 1H), 7.63 (d, J = 1.5 Hz, 1H), 7.86 (d, J = 8.3 Hz, 4H), 8.02 (d, J = 8.9, 1.5 Hz, 1H), 8.50 (d, J = 8.9 Hz, 1H). 13C NMR (125 MHz, CDCl3) δ 21.7 (2C), 28.4 (3C), 41.4, 52.0, 116.2, 117.1, 120.7, 126.1, 126.6, 127.2, 128.6 (4C), 128.8, 129.7 (4C), 136.0 (2C), 138.1, 145.5 (2C), 166.7, 176.7. IR (neat) 1717, 1379, 1360, 1313, 1165 cm–1. MS (ESI) calcd for C29H31N2O7S2 [M + H]+ 583.1567, found 583.1571.
N-(5-Cyano-1-pivaloyl-1H-indol-3-yl)-4-methyl-N-tosylbenzenesulfonamide (2m). White solid, mp 214.0–215.0 °C, 1H NMR (500 MHz, CDCl3) δ 1.42 (s, 9H), 2.49 (s, 6H), 7.17 (d, J = 1.5 Hz, 1H), 7.34 (d, J = 8.0 Hz, 4H), 7.55 (dd, J = 9.0, 1.5 Hz, 1H), 7.64 (s, 1H), 7.83 (d, J = 8.0 Hz, 4H), 8.55 (d, J = 8.5 Hz, 1H). 13C NMR (125 MHz, CDCl3) δ 21.7 (2C), 28.2 (3C), 41.5, 107.6, 115.5, 118.2, 118.8, 123.4, 126.9, 128.5 (4C), 128.7, 129.5, 129.8 (4C), 135.7 (2C), 137.2, 145.8 (2C), 176.6. IR (neat) 2221, 1715, 1363, 1348, 1308, 1169, 1086 cm–1. MS (ESI) calcd for C28H28N3O5S2 [M + H]+ 550.1465, found 550.1470.
N-(5-(1,3-Dioxoisoindolin-2-yl)-1-pivaloyl-1H-indol-3-yl)-4-methyl-N-tosylbenzenesulfonamide (2n). White solid, mp 227.5–228.0 °C, 1H NMR (500 MHz, CDCl3) δ 1.39 (s, 9H), 2.44 (s, 6H), 7.23 (d, J = 2.0 Hz, 1H), 7.38 (d, J = 8.3 Hz, 4H), 7.42 (dd, J = 9.0, 2.0 Hz, 1H), 7.47 (s, 1H), 7.80 (dd, J = 5.5, 3.0 Hz, 2H), 7.92 (d, J = 8.3 Hz, 4H), 7.96 (dd, J = 5.5, 3.0 Hz, 2H), 8.61 (d, J = 9.0 Hz, 1H). 13C NMR (125 MHz, CDCl3) δ 21.7 (2C), 28.4 (3C), 41.3, 115.6, 117.5, 118.0, 123.6 (2C), 124.7, 127.3, 127.9, 128.6 (4C), 128.9, 129.8 (4C), 131.8 (2C), 134.3 (2C), 134.9, 136.0 (2C), 145.3 (2C), 167.1 (2C), 176.6. IR (neat) 1727, 1705, 1375, 1355, 1308, 1167, 1080 cm–1. MS (ESI) calcd for C35H32N3O7S2 [M + H]+ 670.1676, found 670.1678.
4-Methyl-N-(6-methyl-1-pivaloyl-1H-indol-3-yl)-N-tosylbenzenesulfonamide (2o). White solid, mp 203.5–204.2 °C, 1H NMR (500 MHz, CDCl3) δ 1.38 (s, 9H), 2.45 (s, 3H), 2.46 (s, 6H), 6.95 (d, J = 8.0 Hz, 1H), 7.01 (d, J = 8.0 Hz, 1H), 7.29–7.35 (m, 4H), 7.37 (s, 1H), 7.84–7.89 (m, 4H), 8.32 (s, 1H). 13C NMR (125 MHz, CDCl3) δ 21.7 (2C), 21.9, 28.4 (3C), 41.3, 115.8, 117.4, 118.2, 124.6, 125.6, 127.0, 128.6 (4C), 129.6 (4C), 136.1, 136.26, 136.28 (2C), 145.2 (2C), 176.9. IR (neat) 1704, 1371, 1339, 1313, 1163 cm–1. MS (ESI) calcd for C28H30ClN2O5S2 [M + Cl]– 573.1290, found 573.1303.
N-(6-Fluoro-1-pivaloyl-1H-indol-3-yl)-4-methyl-N-tosylbenzenesulfonamide (2p). White solid, mp 215.0–215.5 °C, 1H NMR (500 MHz, CDCl3) δ 1.40 (s, 9H), 2.46 (s, 6H), 6.89–7.02 (m, 2H), 7.32 (d, J = 8.0 Hz, 4H), 7.46 (s, 1H), 7.85 (d, J = 8.0 Hz, 4H), 8.22 (dd, J = 10.3, 2.0 Hz, 1H). 13C NMR (125 MHz, CDCl3) δ 21.7 (2C), 28.4 (3C), 41.3, 104.7 (d, JC–F = 29.8 Hz), 112.6 (d, JC–F = 23.9 Hz), 115.7, 119.4 (d, JC–F = 9.5 Hz), 123.1, 127.8 (d, JC–F = 3.5 Hz), 128.5 (4C), 129.7 (4C), 135.7 (d, JC–F = 13.1 Hz), 136.1 (2C), 145.4 (2C), 161.7 (d, JC–F = 240.9 Hz), 176.6. 19F NMR (471 MHz, CDCl3) δ –115.1. IR (neat) 1707, 1382, 1345, 1315, 1230, 1162 cm–1. MS (ESI) calcd for C27H27ClFN2O5S2 [M + Cl]– 577.1039, found 577.1055.
N-(6-Chloro-1-pivaloyl-1H-indol-3-yl)-4-methyl-N-tosylbenzenesulfonamide (2q). White solid, mp 212.5–213.0 °C, 1H NMR (500 MHz, CDCl3) δ 1.40 (s, 9H), 2.47 (s, 6H), 6.94 (d, J = 8.6 Hz, 1H), 7.14 (dd, J = 8.6, 1.7 Hz, 1H), 7.30–7.35 (m, 4H), 7.46 (s, 1H), 7.82–7.87 (m, 4H), 8.54 (d, J = 1.7 Hz, 1H). 13C NMR (125 MHz, CDCl3) δ 21.7 (2C), 28.4 (3C), 41.3, 115.7, 117.6, 119.4, 124.8, 125.4, 128.1, 128.5 (4C), 129.7 (4C), 132.1, 135.8, 136.1 (2C), 145.4 (2C), 176.6. IR (neat) 1706, 1381, 1360, 1337, 1160, 1083 cm–1. MS (ESI) calcd for C27H28ClN2O5S2 [M + H]+ 559.1123, found 559.1123.
N-(6-Bromo-1-pivaloyl-1H-indol-3-yl)-4-methyl-N-tosylbenzenesulfonamide (2r). White solid, mp 214.0–214.8 °C, 1H NMR (500 MHz, CDCl3) δ 1.39 (s, 9H), 2.47 (s, 6H), 6.90 (d, J = 8.5 Hz, 1H), 7.29 (dd, J = 8.5, 1.5 Hz, 1H), 7.32 (d, J = 8.5 Hz, 4H), 7.44 (s, 1H), 7.85 (d, J = 8.5 Hz, 4H), 8.71 (d, J = 1.5 Hz, 1H). 13C NMR (125 MHz, CDCl3) δ 21.7 (2C), 28.3 (3C), 41.3, 115.7, 119.7, 119.9, 120.4, 125.7, 127.5, 128.0, 128.5 (4C), 129.7 (4C), 136.06 (2C), 136.11, 145.4 (2C), 176.6. IR (neat) 1705, 1380, 1336, 1308, 1159, 658 cm–1. MS (ESI) calcd for C27H27BrClN2O5S2 [M + Cl]– 637.0239, found 637.0256.
N-(6-Methoxy-1-pivaloyl-1H-indol-3-yl)-4-methyl-N-tosylbenzenesulfonamide (2s). White solid, mp 201.0–201.8 °C, 1H NMR (500 MHz, CDCl3) δ 1.40 (s, 9H), 2.46 (s, 6H), 3.86 (s, 3H), 6.80 (dd, J = 8.5, 2.3 Hz, 1H), 6.90 (d, J = 8.5 Hz, 1H), 7.29–7.34 (m, 4H), 7.36 (s, 1H), 7.84–7.89 (m, 4H), 8.07 (d, J = 2.3 Hz, 1H). 13C NMR (125 MHz, CDCl3) δ 21.7 (2C), 28.4 (3C), 41.3, 55.6, 100.8, 114.1, 115.9, 119.1, 120.5, 126.2, 128.5 (4C), 129.6 (4C), 136.3 (2C), 136.7, 145.2 (2C), 159.0, 177.1. IR (neat) 1703, 1378, 1346, 1312, 1286, 1162 cm–1. MS (ESI) calcd for C28H30ClN2O5S2 [M + Cl]– 589.1239, found 589.1257.
N-(4-Fluoro-1-pivaloyl-1H-indol-3-yl)-4-methyl-N-tosylbenzenesulfonamide (2t). White solid, mp 220.0–222.6 °C, 1H NMR (500 MHz, CDCl3) δ 1.42 (s, 9H), 2.46 (s, 6H), 6.85 (dd, J = 10.0, 8.0 Hz, 1H), 7.24–7.30 (m, 1H), 7.30–7.34 (m, 4H), 7.50 (s, 1H), 7.84–7.88 (m, 4H), 8.28 (d, J = 8.5 Hz, 1H). 13C NMR (125 MHz, CDCl3) δ 21.7 (2C), 28.4 (3C), 41.4, 110.2 (d, JC–F = 19.0 Hz), 112.9, 113.3 (d, JC–F = 3.6 Hz), 115.7 (d, JC–F = 17.9 Hz), 126.7 (d, JC–F = 7.3 Hz), 128.0, 128.6 (4C), 129.5 (4C), 136.1(2C), 137.7 (d, JC–F = 7.1 Hz), 145.1 (2C), 154.7 (d, JC–F = 249.1 Hz), 176.7. 19F NMR (471 MHz, CDCl3) δ –122.8. IR (neat) 1707, 1375, 1359, 1309, 1251, 1176 cm–1. MS (ESI) calcd for C27H27ClFN2O5S2 [M + Cl]– 577.1039, found 577.1053.
4-Methyl-N-(7-methyl-1-pivaloyl-1H-indol-3-yl)-N-tosylbenzenesulfonamide (2u). White solid, mp 190.0–191.0 °C, 1H NMR (500 MHz, CDCl3) δ 1.41 (s, 9H), 2.30 (s, 3H), 2.45 (s, 6H), 6.94 (dd, J = 7.5, 1.5 Hz, 1H), 7.06–7.12 (m, 2H), 7.20 (s, 1H), 7.29–7.33 (m, 4H), 7.84–7.89 (m, 4H). 13C NMR (125 MHz, CDCl3) δ 21.2, 21.7 (2C), 28.9 (3C), 42.1, 114.8, 116.6, 123.8, 125.4, 127.5, 128.00, 128.03, 128.6 (4C), 129.5 (4C), 134.9, 136.3 (2C), 145.1 (2C), 178.2. IR (neat) 1715, 1377, 1361, 1303, 1171 cm–1. MS (APCI) calcd for C28H31N2O5S2 [M + H]+ 539.1669, found 539.1670.
N-(5,6-Dichloro-1-pivaloyl-1H-indol-3-yl)-4-methyl-N-tosylbenzenesulfonamide (2v). White solid, mp 223.5–224.2 °C, 1H NMR (500 MHz, CDCl3) δ 1.41 (s, 9H), 2.48 (s, 6H), 6.87 (s, 1H), 7.32–7.36 (m, 4H), 7.52 (s, 1H), 7.82–7.86 (m, 4H), 8.65 (s, 1H). 13C NMR (125 MHz, CDCl3) δ 21.7 (2C), 28.3 (3C), 41.3, 115.0, 119.1, 119.5, 126.4, 128.5 (5C), 129.0, 129.8 (4C), 130.1, 134.0, 135.9 (2C), 145.7 (2C), 176.4. IR (neat) 1710, 1382, 1361, 1330, 1158, 1084 cm–1. MS (ESI) calcd for C27H27Cl2N2O5S2 [M + H]+ 593.0733, found 593.0732.
4-Methyl-N-(2-methyl-1-pivaloyl-1H-indol-3-yl)-N-tosylbenzenesulfonamide (2w). White solid, mp 158.0–159.0 °C, 1H NMR (500 MHz, CDCl3) δ 1.34 (s, 9H), 1.79 (s, 3H), 2.45 (s, 6H), 6.99–7.06 (m, 2H), 7.12–7.18 (m, 1H), 7.21 (d, J = 8.0 Hz, 1H), 7.30 (d, J = 8.5 Hz, 4H), 7.85 (d, J = 8.5 Hz, 4H). 13C NMR (125 MHz, CDCl3) δ 10.8, 21.6 (2C), 28.0 (3C), 44.4, 110.5, 111.7, 118.7, 121.7, 122.8, 125.8, 128.6 (4C), 129.5 (4C), 133.8, 136.7 (2C), 137.9, 145.0 (2C), 185.8. IR (neat) 1710, 1384, 1360, 1313, 1165, 1084 cm–1. MS (APCI) calcd for C28H31N2O5S2 [M + H]+ 539.1669, found 539.1671.
N-(1-Benzoyl-1H-indol-3-yl)-4-methyl-N-tosylbenzenesulfonamide (2x). White solid, mp 214.0–215.0 °C, 1H NMR (500 MHz, CDCl3) δ 2.47 (s, 6H), 7.10 (d, J = 8.0 Hz, 1H), 7.12 (s, 1H), 7.19–7.24 (m, 1H), 7.32 (d, J = 8.5 Hz, 4H), 7.36–7.41 (m, 1H), 7.45–7.51 (m, 2H), 7.59–7.65 (m, 3H), 7.87 (d, J = 8.5 Hz, 4H), 8.37 (d, J = 8.0 Hz, 1H). 13C NMR (125 MHz, CDCl3) δ 21.7 (2C), 116.2, 116.3, 119.0, 1124.5, 125.8, 128.0, 128.59 (4C), 128.64 (2C), 129.3, 129.4 (2C), 129.6 (4C), 132.5, 133.4, 135.0, 136.2 (2C), 145.2 (2C), 168.1. IR (neat) 1694, 1376, 1359, 1325, 1166 cm–1. MS (ESI) calcd for C29H25N2O5S2 [M + H]+ 545.1199, found 545.1205.
4-Methyl-N-tosyl-N-(1-tosyl-1H-indol-3-yl)benzenesulfonamide (2y). White solid, mp 182.0–183.0 °C, 1H NMR (500 MHz, CDCl3) δ 2.35 (s, 3H), 2.44 (s, 6H), 7.04 (d, J = 8.0 Hz, 1H), 7.08–7.13 (m, 1H), 7.23–7.30 (m, 7H), 7.43 (s, 1H), 7.70–7.77 (m, 6H), 7.92 (d, J = 8.5 Hz, 1H). 13C NMR (125 MHz, CDCl3) δ 21.5, 21.7 (2C), 113.5, 116.8, 119.4, 123.9, 125.4, 126.9 (2C), 128.2, 128.3, 128.5 (4C), 129.5 (4C), 130.0 (2C), 133.7, 134.5, 136.0 (2C), 145.3 (2C), 145.5. IR (neat) 1595, 1372, 1355, 1283, 1168, 1086 cm–1. MS (ESI) calcd for C29H27N2O6S3 [M + H]+ 595.1026, found 595.1027.