Metabolomic Profiling of the Desiccation-Tolerant Medicinal Shrub Myrothamnus flabellifolia Indicates Phenolic Variability Across Its Natural Habitat: Implications for Tea and Cosmetics Production
Abstract
:1. Introduction
2. Results
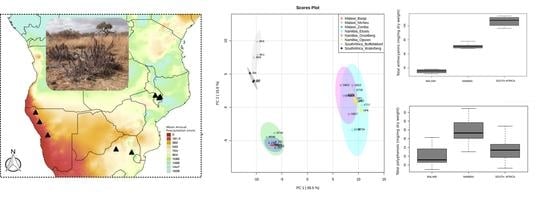
2.1. Total Anthocyanins and Phenolics
2.2. Tentative Identification of Anthocyanins in the Positive Ionisation Mode
2.3. Tentative Identification of Phenolic Acids and Derivatives, Including Hydrolysable Tannins, in the Negative Ionisation Mode
2.4. Tentative Identification of Flavonoids in the Negative Ionisation Mode
2.5. Comparison of the Phenolic Profiles
3. Discussion
4. Materials and Methods
4.1. Plant Material and Sample Preparation
4.2. Total Phenolic and Anthocyanin Assays
4.3. LC-MS/MS Parameters
4.4. Multivariate Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huang, W.Y.; Cai, Y.Z.; Zhang, Y. Natural phenolic compounds from medicinal herbs and dietary plants: Potential use for cancer prevention. Nutr. Cancer 2000, 62, 1–20. [Google Scholar] [CrossRef]
- Mitjavila, M.T.; Moreno, J.J. The effects of polyphenols on oxidative stress and the arachidonic acid cascade. Implications for the prevention/treatment of high prevalence diseases. Biochem. Pharm. 2012, 84, 1113–1122. [Google Scholar] [CrossRef]
- Hertog, M.G.; Kromhout, D.; Aravanis, C.; Blackburn, H.; Buzina, R.; Fidanza, F.; Giampaoli, S.; Jansen, A.; Menotti, A.; Nedeljkovic, S.; et al. Flavonoid intake and long-term risk of coronary heart disease and cancer in the seven countries study. Arch. Intern. Med. 1995, 155, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Tresserra-Rimbau, A.; Lamuela-Raventos, R.M.; Moreno, J.J. Polyphenols, food and pharma. Current knowledge and directions for future research. Biochem. Pharm. 2018, 156, 186–195. [Google Scholar] [CrossRef]
- Porembski, S.; Barthlott, W. Granitic and gneissic outcrops (inselbergs) as centers of diversity for desiccation-tolerant vascular plants. Plant Ecol. 2000, 151, 19–28. [Google Scholar] [CrossRef]
- Gaff, D.F. Desiccation tolerant vascular plants of Southern Africa. Oecologia 1977, 31, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Alpert, P.; Oliver, M.J. Drying without dying. In Desiccation and Survival in Plants: Drying without Dying; Black, M., Pritchard, H.W., Eds.; CABI: Wallingford, UK, 2002; pp. 3–43. [Google Scholar]
- Gaff, D.F.; Oliver, M. The evolution of desiccation tolerance in angiosperm plants: A rare yet common phenomenon. Funct. Plant Biol. 2013, 40, 315–328. [Google Scholar] [CrossRef]
- Kranner, I.; Beckett, R.P.; Wornik, S.; Zorn, M.; Pfeifhofer, H.W. Revival of a resurrection plant correlates with its antioxidant status. Plant J. 2002, 31, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Sherwin, H.W.; Farrant, J.M. Protection mechanisms against excess light in the resurrection plants Craterostigma wilmsii and Xerophyta viscosa. Plant Growth Regul. 1998, 24, 203–210. [Google Scholar] [CrossRef]
- Chalker-Scott, L. Environmental significance of anthocyanins in plant stress responses. Photochem. Photobiol. 1999, 70, 1–9. [Google Scholar] [CrossRef]
- Choinski, J.S., Jr.; Johnson, J.M. Changes in photosynthesis and water status of developing leaves of Brachystegia spiciformis Benth. Tree Physiol. 1993, 13, 17–27. [Google Scholar] [CrossRef]
- Wang, H.; Cao, G.; Prior, R.L. Oxygen radical absorbing capacity of anthocyanins. J. Agric. Food Chem. 1997, 45, 304–309. [Google Scholar] [CrossRef]
- Shin, D.Y.; Lee, W.S.; Lu, J.N.; Kang, M.H.; Ryu, C.H.; Kim, G.Y.; Kang, H.S.; Shin, S.C.; Choi, Y.H. Induction of apoptosis in human colon cancer HCT-116 cells by anthocyanins through suppression of Akt and activation of p38-MAPK. Int. J. Oncol. 2009, 35, 1499–1504. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.M.; Chia, L.S.; Goh, N.K.; Chia, T.F.; Brouillard, R. Analysis and biological activities of anthocyanins. Phytochemistry 2003, 64, 923–933. [Google Scholar] [CrossRef]
- Moore, J.P.; Farrant, J.M.; Lindsey, G.G.; Brandt, W.F. The South African and Namibian populations of the resurrection plant Myrothamnus flabellifolius are genetically distinct and display variation in their galloylquinic acid composition. J. Chem. Ecol. 2005, 31, 2823–2834. [Google Scholar] [CrossRef] [PubMed]
- Kamng’ona, A.; Moore, J.P.; Lindsey, G.; Brandt, W.F. Inhibition of HIV-1 and M-MLV reverse transcriptases by a major polyphenol (3,4,5 tri-O-galloylquinic acid) present in the leaves of the South African resurrection plant, Myrothamnus flabellifolia. J. Enzym. Inhib. Med. Chem. 2011, 26, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Brar, J.; Fultang, N.; Askey, K.; Tettamanzi, M.C.; Peethambaran, B. A novel anti-triple negative breast cancer compound isolated from medicinal herb Myrothamnus flabellifolius. J. Med. Plants Res. 2018, 12, 7–14. [Google Scholar] [CrossRef]
- Gescher, K.; Kühn, J.; Lorentzen, E.; Hafezi, W.; Derksen, A.; Deters, A.; Hensel, A. Proanthocyanidin-enriched extract from Myrothamnus flabellifolia Welw. exerts antiviral activity against herpes simplex virus type 1 by inhibition of viral adsorption and penetration. J. Ethnopharmacol. 2011, 134, 468–474. [Google Scholar] [CrossRef]
- Kwape, T.E.; Majinda, R.R.; Chaturvedi, P. Antioxidant and antidiabetic potential of Myrothamnus flabellifolius found in Botswana. Cogent Biol. 2016, 21, 1275403. [Google Scholar] [CrossRef]
- Moore, J.P.; Westall, K.L.; Ravenscroft, N.; Farrant, J.M.; Lindsey, G.G.; Brandt, W.F. The predominant polyphenol in the leaves of the resurrection plant Myrothamnus flabellifolius, 3,4,5 tri-O-galloylquinic acid, protects membranes against desiccation and free radical-induced oxidation. Biochem. J. 2005, 308, 301–308. [Google Scholar] [CrossRef]
- Engelhardt, C.; Petereit, F.; Anke, J.; Hensel, A. A new arbutin derivative from the herb of Myrothamnus flabellifolia Welw. Phamazie 2007, 62, 558–559. [Google Scholar] [CrossRef]
- Engelhardt, C.; Petereit, F.; Lechtenberg, M.; Liefländer-Wulf, U.; Hensel, A. Qualitative and quantitative phytochemical characterization of Myrothamnus flabellifolia Welw. Fitoterapia 2016, 114, 69–80. [Google Scholar] [CrossRef]
- Anke, J.; Petereit, F.; Engelhardt, C.; Hensel, A. Procyanidins from Myrothamnus flabellifolia. Nat. Prod. Res. 2008, 22, 1237–1248. [Google Scholar] [CrossRef]
- Petereit, F.; Anke, J.; Engelhardt, C.; Hensel, A. Proanthocyanidins from the herb of Myrothamnus flabellifolia Welw. Planta Med. 2006, 72, 134. [Google Scholar] [CrossRef]
- Cheikhyoussef, A.; Summers, R.W.; Kahaka, G.K. Qualitative and quantitative analysis of phytochemical compounds in Namibian Myrothamnus flabellifolius. Int. Sci. Technol. J. Namib. 2015, 5, 71–83. [Google Scholar]
- Koonjul, P.K.; Brandt, W.F.; Lindsey, G.G.; Farrant, J.M. Isolation and characterisation of chloroplasts from Myrothamnus flabellifolius Welw. J. Plant Physiol. 2000, 156, 584–594. [Google Scholar] [CrossRef]
- Moore, J.P.; Ravenscroft, N.; Lindsey, G.G.; Farrant, J.M.; Brandt, W.F. Galloylquinate ester: Anthocyanin complexes in the leaves of the desiccated resurrection plant Myrothamnus flabellifolius. In Proceedings of the Polyphenols Communications: XXII International Conference on Polyphenols 2004, Helsinki, Finland, 25–28 August 2004; pp. 25–28. [Google Scholar]
- Bentley, J.; Moore, J.P.; Farrant, J.M. Metabolomics as a complement to phylogenetics for assessing intraspecific boundaries in the desiccation-tolerant medicinal shrub Myrothamnus flabellifolia (Myrothamnaceae). Phytochemistry 2019, 159, 127–136. [Google Scholar] [CrossRef] [PubMed]
- McKown, R.; Kuroki, G.; Warren, G. Cold responses of Arabidopsis mutants impaired in freezing tolerance. J. Exp. Bot. 1996, 47, 1919–1925. [Google Scholar] [CrossRef]
- Howe, G.T.; Hackett, W.P.; Furnier, G.R.; Klevorn, R.E. Photoperiodic responses of a northern and southern ecotype of black cottonwood. Physiol. Plant. 1995, 93, 695–708. [Google Scholar] [CrossRef]
- Hsieh, T.J.; Liu, T.Z.; Chia, Y.C.; Chern, C.L.; Lu, F.J.; Chuang, M.C.; Mau, S.Y.; Chen, S.H.; Syu, Y.H.; Chen, C.H. Protective effect of methyl gallate from Toona sinensis (Meliaceae) against hydrogen peroxide-induced oxidative stress and DNA damage in MDCK cells. Food Chem. Toxicol. 2004, 42, 843–850. [Google Scholar] [CrossRef]
- Kane, C.J.; Menna, J.H.; Sung, C.C.; Yeh, Y.C. Methyl gallate, methyl-3, 4, 5-trihydroxybenzoate, is a potent and highly specific inhibitor of herpes simplex virus in vitro. II. Antiviral activity of methyl gallate and its derivatives. Biosci. Rep. 1988, 8, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Quideau, S.; Jourdes, M.; Lefeuvre, D.; Montaudon, D.; Saucier, C.; Glories, Y.; Pardon, P.; Pourquier, P. The chemistry of wine polyphenolic C-glycosidic ellagitannins targeting human topoisomerase II. Chem.-Eur. J. 2005, 11, 6503–6513. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Engelhardt, U.H.; Thräne, C.; Maiwald, B.; Stark, J. Determination of flavonol glycosides in green tea, oolong tea and black tea by UHPLC compared to HPLC. Food Chem. 2015, 183, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Migas, P.; Krauze-Baranowska, M. The significance of arbutin and its derivatives in therapy and cosmetics. Phytochem. Lett. 2015, 13, 35–40. [Google Scholar] [CrossRef]
- Maier, T.S.; Kuhn, J.; Müller, C. Proposal for field sampling of plants and processing in the lab for environmental metabolic fingerprinting. Plant Methods 2010, 2010 61, 6. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Method Enzym. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef] [PubMed]
- Neff, M.M.; Chory, J. Genetic Interactions between Phytochrome A, Phytochrome B, and Cryptochrome 1 during Arabidopsis Development. Plant Physiol. 1998, 118, 27–35. [Google Scholar] [CrossRef]
- Giusti, M.M.; Rodríguez-Saona, L.E.; Griffin, D.; Wrolstad, R.E. Electrospray and tandem mass spectroscopy as tools for anthocyanin characterization. J. Agric. Food Chem. 1999, 47, 4657–4664. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 1 June 2014).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2009. [Google Scholar]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis. Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef]
- Smith, C.A.; Want, E.J.; O’Maille, G.; Abagyan, R.; Siuzdak, G. XCMS: Processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching and identification. Anal. Chem. 2006, 78, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Tautenhahn, R.; Boettcher, C.; Neumann, S. Highly sensitive feature detection for high resolution LC/MS. BMC Bioinform. 2008, 9, 504. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the extracts are not available. |




| No. | Tentative Identification in Negative Ionisation Mode | m/z [M − H]− | RT | MS |
|---|---|---|---|---|
| 1 | Quinic acid | 191.0555 | 1.7 | 173, 161, 133 |
| 2 | Naringenin or arbutin isomer | 271.0812 | 1.8 | 161, 151, 125, 119 |
| 3 | Quinic acid derivative | 317.0865 | 1.8 | 191, 179, 133 |
| 4 | Dimer of quinic acid/caffeic acid glycoside | 683.2248 | 1.9 | 341, 191 |
| 5 | Quinic acid derivative | 317.0873 | 5.7 | 191, 135 271, 255 |
| 6 | Arbutin | 271.0817 | 5.7 | 161, 109 |
| 7 | Gallic acid | 169.0137 | 6.3 | 125 |
| 8 | 3/4/5-O-galloylquinic acid | 343.0661 | 7.6 | 301, 191, 173, 169, 125 |
| 9 | Quinic acid derivative | 405.1028 | 9.2 | 191, 173 |
| 10 | 3,4/3,5-Di-O-galloylquinic acid | 495.0777 | 11.4 | 343, 325, 191, 169, 125 |
| 11 | Methyl gallate | 183.0297 | 11.7 | 124 |
| 12 | Quinic acid derivative | 423.0925 | 11.9 | 257, 191 |
| 13 | 1,2,3/1,4,6-Tri-O-galloyl-glucose | 635.0884 | 13.4 | 465, 271, 169, 125 |
| 14 | 3,4,5-Tri-O-galloylquinic acid | 647.0884 | 14.0 | 495, 343, 191, 169, 125 |
| 15 | Ellagic acid derivative | 431.1916 | 14.3 | 301, 169, 153, 125 |
| 16 | Gallocatechin/epigallocatechin gallate | 457.077 | 14.4 | 305, 169, 165, 125 |
| 17 | Galloylquinic acid derivative | 784.5787 | 14.8 | 647, 481, 301, 191, 169, 125 |
| 18 | Quinic acid derivative | 860.3122 | 15.4 | 709, 477, 301, 191 |
| 19 | Penta-galloylquinate ester | 937.0837 | 16.3 | 799, 468, 301 169, 125 |
| 20 | Ellagic acid derivative | 468.0443 | 16.6 | 301, 169, 125 |
| 21 | Quercetin-3-O-hexose-gallate | 615.1082 | 17.3 | 463, 300, 169, 151, 125 |
| 22 | Ellagic acid derivative | 441.0854 | 17.7 | 301 |
| 23 | Ellagic acid | 300.9985 | 18.0 | 229, 169, 125 |
| 24 | Quercetin glucoside | 463.0876 | 18.3 | 301 |
| 25 | Miquelianin (quercetin-3-O-glucuronide) | 477.0671 | 18.4 | 301, 273, 255, 179, 151 |
| 26 | Quercetin derivative | 469.0521 | 18.6 | 301 |
| 27 | Galloyl quercetin-3-O-rhamnoside | 599.1032 | 18.7 | 447, 301, 171, 169, 125 |
| 28 | Digalloylglucose | 483.1863 | 19.8 | 315, 301, 169, 125 |
| 29 | Unknown phenolic | 711.3957 | 21.0 | 647, 481, 301, 191, 169, 125 |
| 30 | Quercitrin (quercetin 3-rhamnoside) | 447.0923 | 24.0 | 301, 300, 271, 255, 179 |
| 31 | Methylellagic acid pentose | 447.0924 | 24.1 | 315 |
| 32 | Isorhamnetin(3-O-methylquercetin) | 315.0504 | 24.2 | 300, 271, 255, 243, 151 |
| No. | Tentative Identification in Positive Ionisation Mode | m/z [M + H]+ | RT | MS |
| 1 | Delphinidin-3-O-glucoside (myrtillin) | 465.1030 | 12.8 | 303, 257, 229 |
| 2 | Delphinidin-3-(6-O-coumaroyl)glucoside/Delphinidin rutinoside/Quercetin 3-O-rutinoside (rutin) | 611.1616 | 12.8 | 465, 303, 153 |
| 3 | Quercetin-7-O-glucuronide (not an anthocyanin) | 479.0826 | 13.8 | 303 |
| 4 | Cyanidin-3-O-rutinoside | 595.1667 | 14.8 | 449, 287 |
| 5 | Delphinidin-3-O-arabinoside | 435.0910 | 15.0 | 303 |
| 6 | Cyanidin-3-O-arabinoside | 419.1040 | 17.3 | 287 |
| 7 | Peonidin-3-O-glucoside/galactoside | 463.1242 | 21.0 | 301, 153 |
| 8 | Petunidin-3-O-arabinoside | 449.1079 | 22.4 | 317 |
| 9 | Delphinidin-3-acetylglucoside | 507.3283 | 28.8 | 463 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bentley, J.; Moore, J.P.; Farrant, J.M. Metabolomic Profiling of the Desiccation-Tolerant Medicinal Shrub Myrothamnus flabellifolia Indicates Phenolic Variability Across Its Natural Habitat: Implications for Tea and Cosmetics Production. Molecules 2019, 24, 1240. https://doi.org/10.3390/molecules24071240
Bentley J, Moore JP, Farrant JM. Metabolomic Profiling of the Desiccation-Tolerant Medicinal Shrub Myrothamnus flabellifolia Indicates Phenolic Variability Across Its Natural Habitat: Implications for Tea and Cosmetics Production. Molecules. 2019; 24(7):1240. https://doi.org/10.3390/molecules24071240
Chicago/Turabian StyleBentley, Joanne, John P. Moore, and Jill M. Farrant. 2019. "Metabolomic Profiling of the Desiccation-Tolerant Medicinal Shrub Myrothamnus flabellifolia Indicates Phenolic Variability Across Its Natural Habitat: Implications for Tea and Cosmetics Production" Molecules 24, no. 7: 1240. https://doi.org/10.3390/molecules24071240
APA StyleBentley, J., Moore, J. P., & Farrant, J. M. (2019). Metabolomic Profiling of the Desiccation-Tolerant Medicinal Shrub Myrothamnus flabellifolia Indicates Phenolic Variability Across Its Natural Habitat: Implications for Tea and Cosmetics Production. Molecules, 24(7), 1240. https://doi.org/10.3390/molecules24071240