Antiproliferative Aspidosperma-Type Monoterpenoid Indole Alkaloids from Bousigonia mekongensis Inhibit Tubulin Polymerization
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Antiproliferative Activity of Compounds 1–14
2.3. Biological Activity Comparison
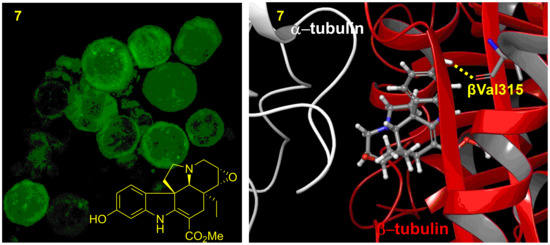
2.4. Mechanisms of Action of 3, 4, 6, 7, and 13 in KB-VIN Cells
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Antiproliferative Activity Assay
3.5. Tubulin Assays
3.6. Cell Cycle Analysis
3.7. Immunofluorescence Staining
3.8. Computer Modeling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Disclaimer
Conflicts of Interest
References
- Dumontet, C.; Jordan, M.A. Microtubule-binding agents: A dynamic field of cancer therapeutics. Nat. Rev. Drug Discov. 2010, 9, 790–803. [Google Scholar] [CrossRef]
- Perez, E.A. Microtubule inhibitors: Differentiating tubulin-inhibiting agents based on mechanisms of action, clinical activity, and resistance. Mol. Cancer Ther. 2009, 8, 2086–2095. [Google Scholar] [CrossRef] [Green Version]
- Szakács, G.; Hall, M.D.; Gottesman, M.M.; Boumendjel, A.; Kachadourian, R.; Day, B.J.; Baubichon-Cortay, H.; Pietro, A.D. Targeting the Achilles heel of multidrug-resistant cancer by exploiting the fitness cost of resistance. Chem. Rev. 2014, 114, 5753–5774. [Google Scholar] [CrossRef]
- Eckford, P.D.; Sharom, F.J. ABC efflux pump-based resistance to chemotherapy drugs. Chem. Rev. 2009, 109, 2989–3011. [Google Scholar] [CrossRef] [PubMed]
- Kavellaris, M. Microtubules and resistance to tubulin-binding agents. Nat. Rev. Cancer 2010, 10, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Dewick, P.M. Alkaloids. In Medicinal Natural Products: A Biosynthetic Approach, 3rd ed.; John Wiley & Sons Ltd.: Chichester, UK, 2009; pp. 369–380. ISBN 978-0-470-74168-9. [Google Scholar]
- Martino, E.; Casamassima, G.; Castiglione, S.; Cellupica, E.; Pantalone, S.; Papagni, F.; Rui, M.; Siciliano, A.M.; Collina, S. Vinca alkaloids and analogues as anti-cancer agents: Looking back, peering ahead. Bioorg. Med. Chem. Lett. 2018, 28, 2816–2826. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.H.; Hiraku, O.; Komiyama, K.; Kam, T.S. Jerantinines A–G, cytotoxic Aspidosperma alkaloids from Tabernaemontana corymbosa. J. Nat. Prod. 2008, 71, 1591–1594. [Google Scholar] [CrossRef] [PubMed]
- Frei, R.; Staedler, D.; Raja, A.; Franke, R.; Sasse, F.; Gerber-Lemaire, S.; Waser, J. Total synthesis and biological evaluation of Jerantinine E. Angew. Chem. Int. Ed. 2013, 52, 13373–13376. [Google Scholar] [CrossRef]
- Raja, V.J.; Lim, K.H.; Leong, C.O.; Kam, T.S.; Bradshaw, T.D. Novel antitumor indole alkaloid, Jerantinine A, evokes potent G2/M cell cycle arrest targeting microtubules. Invest New Drugs 2014, 32, 838–850. [Google Scholar] [CrossRef] [PubMed]
- Qazzaz, M.E.; Raja, V.J.; Lim, K.H.; Kam, T.S.; Lee, J.B.; Gershkovich, P.; Bradshaw, T.D. In vitro anticancer properties and biological evaluation of novel natural alkaloid jerantinine B. Cancer Lett. 2016, 370, 185–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.T.; Leeuwenberg, J.M.; Middleton, D.J. Flora of China; Science Press: Beijing, China, 1995; Volume 16, p. 150. [Google Scholar]
- Fu, Y.H.; He, H.P.; Di, Y.T.; Li, S.L.; Zhang, Y.; Hao, X.J. Mekongenines A and B, two new alkaloids from Bousigonia mekongensis. Tetrahedron Lett. 2012, 53, 3642–3646. [Google Scholar] [CrossRef]
- Fu, Y.H.; Di, Y.T.; He, H.P.; Li, S.L.; Zhang, Y.; Hao, X.J. Angustifonines A and B, cytotoxic bisindole alkaloids from Bousigonia angustifolia. J. Nat. Prod. 2014, 77, 56–62. [Google Scholar] [CrossRef]
- Fu, Y.H.; Li, S.L.; Li, S.F.; He, H.P.; Di, Y.T.; Zhang, Y.; Hao, X.J. Cytotoxic eburnamine-aspidospermine type bisindole alkaloids from Bousigonia mekongensis. Fitoterapia 2014, 98, 45–52. [Google Scholar] [CrossRef]
- Baassou, S.; Mehri, H.; Plat, M. Plants of New Caledonia. Part 49. Alkaloids of Melodinus aeneus. Phytochemistry 1978, 17, 1449–1450. [Google Scholar] [CrossRef]
- Fahn, W.; Kaiser, V.; Schübel, H.; Stöckigt, J.; Danieli, B. Catharanthus roseus enzyme mediated synthesis of 3-hydroxyvoafrine A and B—A simple route to the voafrines. Phytochemistry 1990, 29, 129–133. [Google Scholar] [CrossRef]
- Liu, Y.P.; Li, Y.; Cai, X.H.; Li, X.Y.; Kong, L.M.; Cheng, G.G.; Luo, X.D. Melodinines M-U, cytotoxic alkaloids from Melodinus suaveolens. J. Nat. Prod. 2012, 75, 220–224. [Google Scholar] [CrossRef]
- Nair, C.P.N.; Pillay, P.P. Lochnericine. A new alkaloid from Lochnera rosea. Tetrahedron 1959, 6, 89–93. [Google Scholar] [CrossRef]
- Guo, L.W.; Zhou, Y.L. Alkaloids from Melodinus hemsleyanus. Phytochemistry 1993, 34, 563–566. [Google Scholar] [CrossRef]
- Men-Olivier, L.L.; Richard, B.; Men, J.L. Alcaloїdes des grains du Pandaca retusa. Phytochemistry 1993, 34, 563–566. [Google Scholar]
- Feng, T.; Li, Y.; Cai, X.H.; Gong, X.; Liu, Y.P.; Zhang, R.T.; Zhang, X.Y.; Tan, Q.G.; Luo, X.D. Monoterpenoid indole alkaloids from Alstonia yunnanensis. J. Nat. Prod. 2009, 72, 1836–1841. [Google Scholar] [CrossRef]
- Langlois, N.; Andriamialisoa, R.Z. Studies on vindolinine. 6. Partial synthesis of aspidospermane-type alkaloids. J. Org. Chem. 1979, 44, 2468–2471. [Google Scholar] [CrossRef]
- Kutney J., P.; Choi, L.S.L.; Kolodziejczyk, P.; Sleigh, S.K.; Stuart, K.L.; Worth, B.R.; Kurz, W.G.W.; Chatson, K.B.; Constabel, F. Alkaloid production in Catharanthus roseus cell cultures: Isolation and characterization of alkaloids from one cell line. Phytochemistry 1980, 19, 2589–2595. [Google Scholar] [CrossRef]
- Smedley, C.J.; Stanley, P.A.; Qazzaz, M.E.; Prota, A.E.; Olieric, N.; Collins, H.; Eastman, H.; Barrow, A.S.; Lim, K.H.; Kam, T.S.; et al. Sustainable syntheses of (-)-jerantinines A & E and structural characterisation of the jerantinine-tubulin complex at the colchicine binding Site. Sci. Rep. 2018, 8, 10617. [Google Scholar] [PubMed]
- Nakagawa-Goto, K.; Oda, A.; Hamel, E.; Ohkoshi, E.; Lee, K.H.; Goto, M. Development of a novel class of tubulin inhibitor from desmosdumotin B with a hydroxylated bicyclic B-ring. J. Med. Chem. 2015, 58, 2378–2389. [Google Scholar] [CrossRef]
- Hamel, E. Evaluation of antimitotic agents by quantitative comparisons of their effects on the polymerization of purified tubulin. Cell Biochem. Biophys. 2003, 38, 1–22. [Google Scholar] [CrossRef]
- Verdier-Pinard, P.; Lai, J.Y.; Yoo, H.D.; Yu, J.; Marquez, B.; Nagle, D.G.; Nambu, M.; White, J.D.; Falck, J.R.; Gerwick, W.H.; et al. Structure−activity analysis of the interaction of curacin A, the potent colchicine site antimitotic agent, with tubulin and effects of analogs on the growth of MCF-7 breast cancer cells. Mol. Pharmacol. 1998, 53, 62–76. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.; Willett, P.; Glen, R.C. Molecular recognition of receptor sites using a genetic algorithm with a description of desolvation. J. Mol. Biol. 1995, 245, 43–53. [Google Scholar] [CrossRef]
- Ravelli, R.B.; Gigant, B.; Curmi, P.A.; Jourdain, I.; Lachkar, S.; Sobel, A.; Knossow, M. Insight into tubulin regulation from a complex with colchicine and a stathmin-like domain. Nature 2004, 428, 198–202. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09 (Revision B.01); Gaussian, Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
Sample Availability: Samples of the compounds 1–14 are available from the authors. |




| Compound | IC50 (μM) a | Tubulin Assay | |||||
|---|---|---|---|---|---|---|---|
| A549 | MDA-MB-231 | KB | KB-VIN | MCF-7 | ITAd (μM) | ICBe (%) | |
| 1 | 6.9 ± 0.1 | 5.9 ± 0.0 | 5.5 ± 0.0 | 7.4 ± 0.3 | 7.2 ± 0.4 | NTb | NT |
| 2 | >40 | >40 | >40 | >40 | >40 | NAc | NA |
| 3 | 0.6 ± 0.0 | 0.9 ± 0.0 | 0.6 ± 0.0 | 0.6 ± 0.1 | 2.2 ± 0.2 | NT | NT |
| 4 | 5.6 ± 0.0 | 8.2 ± 0.8 | 5.8 ± 0.3 | 6.3 ± 0.2 | 10.0 ± 0.9 | NT | NT |
| 5 | >40 | >40 | >40 | >40 | >40 | NA | NA |
| 6 | 0.5 ± 0.0 | 0.8 ± 0.0 | 0.5 ± 0.0 | 0.5 ± 0.0 | 0.8 ± 0.0 | 0.7 ± 0.0 | 54 ± 0.8 |
| 7 | 5.6 ± 0.1 | 9.8 ± 0.9 | 5.4 ± 0.3 | 6.1 ± 0.4 | 10.8 ± 0.8 | 4.6 ± 0.1 | 35 ± 1 |
| 8 | 27.7 ± 0.1 | 34.5 ± 0.2 | >40 | >40 | >40 | NA | NA |
| 9 | 0.8 ± 0.0 | 6.0 ± 0.8 | 0.9 ± 0.0 | 0.7 ± 0.0 | 10.2 ± 0.5 | NT | NT |
| 10 | >40 | >40 | >40 | >40 | >40 | NA | NA |
| 11 | >40 | >40 | 26.5 ± 2.5 | >40 | >40 | NA | NA |
| 12 | 5.2 ± 0.1 | 6.8 ± 1.1 | 5.4 ± 0.4 | 5.8 ± 0.1 | 8.2 ± 0.9 | NT | NT |
| 13 | 0.7 ± 0.1 | 0.9 ± 0.0 | 0.7 ± 0.0 | 0.7 ± 0.0 | 6.7 ± 0.1 | NT | NT |
| 14 | >40 | >40 | >40 | >40 | >40 | NA | NA |
| VIN (nM) | 22.9 ± 2.4 | 32.0 ± 0.5 | 4.4 ± 0.1 | 2479.2 ± 28.2 | 7.3 ± 0.2 | NT | NT |
| PXL (nM) | 4.5 ± 0.9 | 7.0 ± 0.9 | 3.7 ± 1.1 | 2357.7 ± 59.5 | 8.8 ± 1.0 | NT | NT |
| CA-4 (nM) | 5.5 ± 0.1 | 8.2 ± 0.5 | 3.6 ± 0.1 | 3.8 ± 0.1 | 487.4 ± 11 | 0.7 ± 0.0 | 100 ± 0.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Goto, M.; Oda, A.; Hsu, P.-L.; Guo, L.-L.; Fu, Y.-H.; Morris-Natschke, S.L.; Hamel, E.; Lee, K.-H.; Hao, X.-J. Antiproliferative Aspidosperma-Type Monoterpenoid Indole Alkaloids from Bousigonia mekongensis Inhibit Tubulin Polymerization. Molecules 2019, 24, 1256. https://doi.org/10.3390/molecules24071256
Zhang Y, Goto M, Oda A, Hsu P-L, Guo L-L, Fu Y-H, Morris-Natschke SL, Hamel E, Lee K-H, Hao X-J. Antiproliferative Aspidosperma-Type Monoterpenoid Indole Alkaloids from Bousigonia mekongensis Inhibit Tubulin Polymerization. Molecules. 2019; 24(7):1256. https://doi.org/10.3390/molecules24071256
Chicago/Turabian StyleZhang, Yu, Masuo Goto, Akifumi Oda, Pei-Ling Hsu, Ling-Li Guo, Yan-Hui Fu, Susan L. Morris-Natschke, Ernest Hamel, Kuo-Hsiung Lee, and Xiao-Jiang Hao. 2019. "Antiproliferative Aspidosperma-Type Monoterpenoid Indole Alkaloids from Bousigonia mekongensis Inhibit Tubulin Polymerization" Molecules 24, no. 7: 1256. https://doi.org/10.3390/molecules24071256
APA StyleZhang, Y., Goto, M., Oda, A., Hsu, P. -L., Guo, L. -L., Fu, Y. -H., Morris-Natschke, S. L., Hamel, E., Lee, K. -H., & Hao, X. -J. (2019). Antiproliferative Aspidosperma-Type Monoterpenoid Indole Alkaloids from Bousigonia mekongensis Inhibit Tubulin Polymerization. Molecules, 24(7), 1256. https://doi.org/10.3390/molecules24071256