Could Polyphenols Help in the Control of Rheumatoid Arthritis?
Abstract
:1. Introduction
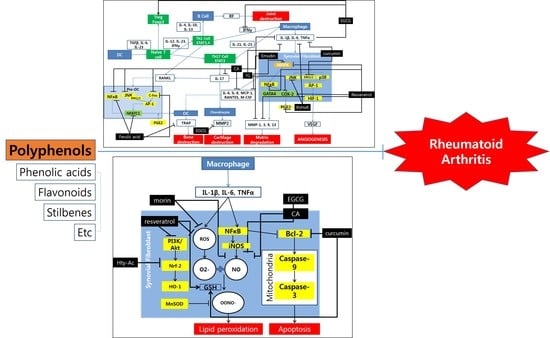
2. Polyphenols and Rheumatoid Arthritis
2.1. Phenolic Acids
2.2. Stilbenes
2.3. Flavonoids
2.4. Other Compounds
3. Discussion
4. Methods
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Smolen, J.S.; Aletaha, D.; McInnes, I.B. Rheumatoid arthritis. Lancet 2016, 388, 2023–2038. [Google Scholar] [CrossRef]
- Firestein, G.S. Evolving concepts of rheumatoid arthritis. Nature 2003, 423, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Disease, G.B.D.; Injury, I.; Prevalence, C. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1545–1602. [Google Scholar] [CrossRef]
- Burmester, G.R.; Pope, J.E. Novel treatment strategies in rheumatoid arthritis. Lancet 2017, 389, 2338–2348. [Google Scholar] [CrossRef]
- McInnes, I.B.; Schett, G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 2011, 365, 2205–2219. [Google Scholar] [CrossRef] [PubMed]
- McInnes, I.B.; Schett, G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat. Rev. Immunol. 2007, 7, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Viatte, S.; Plant, D.; Raychaudhuri, S. Genetics and epigenetics of rheumatoid arthritis. Nat. Rev. Rheumatol 2013, 9, 141–153. [Google Scholar] [CrossRef]
- Choy, E. Understanding the dynamics: Pathways involved in the pathogenesis of rheumatoid arthritis. Rheumatology 2012, 51 (Suppl. 5), v3–v11. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Doss, H.M.; Samarpita, S.; Ganesan, R.; Rasool, M. Ferulic acid, a dietary polyphenol suppresses osteoclast differentiation and bone erosion via the inhibition of RANKL dependent NF-kappaB signalling pathway. Life Sci. 2018. [Google Scholar] [CrossRef] [PubMed]
- Paskova, L.; Kuncirova, V.; Ponist, S.; Mihalova, D.; Nosal, R.; Harmatha, J.; Hradkova, I.; Cavojsky, T.; Bilka, F.; Siskova, K.; et al. Effect of N-Feruloylserotonin and Methotrexate on Severity of Experimental Arthritis and on Messenger RNA Expression of Key Proinflammatory Markers in Liver. J. Immunol. Res. 2016, 2016, 7509653. [Google Scholar] [CrossRef]
- Lee, S.H.; Park, H.H.; Kim, J.E.; Kim, J.A.; Kim, Y.H.; Jun, C.D.; Kim, S.H. Allose gallates suppress expression of pro-inflammatory cytokines through attenuation of NF-kappaB in human mast cells. Planta Med. 2007, 73, 769–773. [Google Scholar] [CrossRef]
- Kwak, S.C.; Lee, C.; Kim, J.Y.; Oh, H.M.; So, H.S.; Lee, M.S.; Rho, M.C.; Oh, J. Chlorogenic acid inhibits osteoclast differentiation and bone resorption by down-regulation of receptor activator of nuclear factor kappa-B ligand-induced nuclear factor of activated T cells c1 expression. Biol. Pharm. Bull. 2013, 36, 1779–1786. [Google Scholar] [CrossRef]
- Pragasam, S.J.; Venkatesan, V.; Rasool, M. Immunomodulatory and anti-inflammatory effect of p-coumaric acid, a common dietary polyphenol on experimental inflammation in rats. Inflammation 2013, 36, 169–176. [Google Scholar] [CrossRef]
- Neog, M.K.; Joshua Pragasam, S.; Krishnan, M.; Rasool, M. p-Coumaric acid, a dietary polyphenol ameliorates inflammation and curtails cartilage and bone erosion in the rheumatoid arthritis rat model. Biofactors 2017, 43, 698–717. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.Y.; Lee, E.G.; Lee, H.; Cho, I.J.; Choi, Y.J.; Sung, M.S.; Yoo, H.G.; Yoo, W.H. Kaempferol inhibits IL-1beta-induced proliferation of rheumatoid arthritis synovial fibroblasts and the production of COX-2, PGE2 and MMPs. Int. J. Mol. Med. 2013, 32, 971–977. [Google Scholar] [CrossRef]
- Sirerol, J.A.; Rodriguez, M.L.; Mena, S.; Asensi, M.A.; Estrela, J.M.; Ortega, A.L. Role of Natural Stilbenes in the Prevention of Cancer. Oxid. Med. Cell. Longev. 2016, 2016, 3128951. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Song, X.; Cao, W.; Lu, J.; Wang, X.; Wang, G.; Wang, Z.; Chen, X. Autophagy and mitochondrial dysfunction in adjuvant-arthritis rats treatment with resveratrol. Sci. Rep. 2016, 6, 32928. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.H.; Hsu, L.F.; Lee, C.W.; Chiang, Y.C.; Lee, M.H.; How, J.M.; Wu, C.M.; Huang, C.L.; Lee, I.T. Resveratrol inhibits urban particulate matter-induced COX-2/PGE2 release in human fibroblast-like synoviocytes via the inhibition of activation of NADPH oxidase/ROS/NF-kappaB. Int. J. Biochem. Cell Biol. 2017, 88, 113–123. [Google Scholar] [CrossRef]
- Tian, J.; Chen, J.W.; Gao, J.S.; Li, L.; Xie, X. Resveratrol inhibits TNF-alpha-induced IL-1beta, MMP-3 production in human rheumatoid arthritis fibroblast-like synoviocytes via modulation of PI3kinase/Akt pathway. Rheumatol. Int. 2013, 33, 1829–1835. [Google Scholar] [CrossRef]
- Khojah, H.M.; Ahmed, S.; Abdel-Rahman, M.S.; Elhakeim, E.H. Resveratrol as an effective adjuvant therapy in the management of rheumatoid arthritis: A clinical study. Clin. Rheumatol. 2018, 37, 2035–2042. [Google Scholar] [CrossRef] [PubMed]
- Xuzhu, G.; Komai-Koma, M.; Leung, B.P.; Howe, H.S.; McSharry, C.; McInnes, I.B.; Xu, D. Resveratrol modulates murine collagen-induced arthritis by inhibiting Th17 and B-cell function. Ann. Rheum. Dis. 2012, 71, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Wahba, M.G.; Messiha, B.A.; Abo-Saif, A.A. Protective effects of fenofibrate and resveratrol in an aggressive model of rheumatoid arthritis in rats. Pharm. Biol. 2016, 54, 1705–1715. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Kim, K.; Vance, T.M.; Chun, O.K. Greater flavonoid intake is associated with improved CVD risk factors in US adults. Br. J. Nutr. 2016, 115, 1481–1488. [Google Scholar] [CrossRef] [PubMed]
- Mulvihill, E.E.; Burke, A.C.; Huff, M.W. Citrus Flavonoids as Regulators of Lipoprotein Metabolism and Atherosclerosis. Annu. Rev. Nutr. 2016, 36, 275–299. [Google Scholar] [CrossRef]
- Kometani, T.; Fukuda, T.; Kakuma, T.; Kawaguchi, K.; Tamura, W.; Kumazawa, Y.; Nagata, K. Effects of alpha-glucosylhesperidin, a bioactive food material, on collagen-induced arthritis in mice and rheumatoid arthritis in humans. Immunopharmacol. Immunotoxicol. 2008, 30, 117–134. [Google Scholar] [CrossRef]
- He, Y.H.; Zhou, J.; Wang, Y.S.; Xiao, C.; Tong, Y.; Tang, J.C.; Chan, A.S.; Lu, A.P. Anti-inflammatory and anti-oxidative effects of cherries on Freund’s adjuvant-induced arthritis in rats. Scand. J. Rheumatol. 2006, 35, 356–358. [Google Scholar] [CrossRef]
- Kim, J.E.; Son, J.E.; Jung, S.K.; Kang, N.J.; Lee, C.Y.; Lee, K.W.; Lee, H.J. Cocoa polyphenols suppress TNF-alpha-induced vascular endothelial growth factor expression by inhibiting phosphoinositide 3-kinase (PI3K) and mitogen-activated protein kinase kinase-1 (MEK1) activities in mouse epidermal cells. Br. J. Nutr. 2010, 104, 957–964. [Google Scholar] [CrossRef]
- Ahmed, S.; Pakozdi, A.; Koch, A.E. Regulation of interleukin-1beta-induced chemokine production and matrix metalloproteinase 2 activation by epigallocatechin-3-gallate in rheumatoid arthritis synovial fibroblasts. Arthritis Rheum. 2006, 54, 2393–2401. [Google Scholar] [CrossRef]
- Yun, H.J.; Yoo, W.H.; Han, M.K.; Lee, Y.R.; Kim, J.S.; Lee, S.I. Epigallocatechin-3-gallate suppresses TNF-alpha -induced production of MMP-1 and -3 in rheumatoid arthritis synovial fibroblasts. Rheumatol. Int. 2008, 29, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Jung, Y.O.; Ryu, J.G.; Oh, H.J.; Son, H.J.; Lee, S.H.; Kwon, J.E.; Kim, E.K.; Park, M.K.; Park, S.H.; et al. Epigallocatechin-3-gallate ameliorates autoimmune arthritis by reciprocal regulation of T helper-17 regulatory T cells and inhibition of osteoclastogenesis by inhibiting STAT3 signaling. J. Leukoc. Biol. 2016, 100, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Min, S.Y.; Yan, M.; Kim, S.B.; Ravikumar, S.; Kwon, S.R.; Vanarsa, K.; Kim, H.Y.; Davis, L.S.; Mohan, C. Green Tea Epigallocatechin-3-Gallate Suppresses Autoimmune Arthritis Through Indoleamine-2,3-Dioxygenase Expressing Dendritic Cells and the Nuclear Factor, Erythroid 2-Like 2 Antioxidant Pathway. J. Inflamm. 2015, 12, 53. [Google Scholar] [CrossRef] [PubMed]
- Leichsenring, A.; Backer, I.; Furtmuller, P.G.; Obinger, C.; Lange, F.; Flemmig, J. Long-Term Effects of (−)-Epigallocatechin Gallate (EGCG) on Pristane-Induced Arthritis (PIA) in Female Dark Agouti Rats. PLoS ONE 2016, 11, e0152518. [Google Scholar] [CrossRef] [PubMed]
- Morinobu, A.; Biao, W.; Tanaka, S.; Horiuchi, M.; Jun, L.; Tsuji, G.; Sakai, Y.; Kurosaka, M.; Kumagai, S. (−)-Epigallocatechin-3-gallate suppresses osteoclast differentiation and ameliorates experimental arthritis in mice. Arthritis Rheum. 2008, 58, 2012–2018. [Google Scholar] [CrossRef]
- Oka, Y.; Iwai, S.; Amano, H.; Irie, Y.; Yatomi, K.; Ryu, K.; Yamada, S.; Inagaki, K.; Oguchi, K. Tea polyphenols inhibit rat osteoclast formation and differentiation. J. Pharmacol. Sci. 2012, 118, 55–64. [Google Scholar] [CrossRef]
- Lee, J.D.; Huh, J.E.; Jeon, G.; Yang, H.R.; Woo, H.S.; Choi, D.Y.; Park, D.S. Flavonol-rich RVHxR from Rhus verniciflua Stokes and its major compound fisetin inhibits inflammation-related cytokines and angiogenic factor in rheumatoid arthritis fibroblast-like synovial cells and in vivo models. Int. Immunopharmacol. 2009, 9, 268–276. [Google Scholar] [CrossRef]
- Rathi, B.; Bodhankar, S.; Mohan, V.; Thakurdesai, P. Ameliorative Effects of a Polyphenolic Fraction of Cinnamomum zeylanicum L. Bark in Animal Models of Inflammation and Arthritis. Sci. Pharm. 2013, 81, 567–589. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Q.; Jin, S.; He, D.; Zhao, S.; Liu, S. Genistein modulate immune responses in collagen-induced rheumatoid arthritis model. Maturitas 2008, 59, 405–412. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, J.; He, P.; Li, W.; Zhang, Q.; Li, N.; Sun, T. Genistein inhibit cytokines or growth factor-induced proliferation and transformation phenotype in fibroblast-like synoviocytes of rheumatoid arthritis. Inflammation 2012, 35, 377–387. [Google Scholar] [CrossRef]
- Umar, S.; Kumar, A.; Sajad, M.; Zargan, J.; Ansari, M.; Ahmad, S.; Katiyar, C.K.; Khan, H.A. Hesperidin inhibits collagen-induced arthritis possibly through suppression of free radical load and reduction in neutrophil activation and infiltration. Rheumatol. Int. 2013, 33, 657–663. [Google Scholar] [CrossRef]
- Decendit, A.; Mamani-Matsuda, M.; Aumont, V.; Waffo-Teguo, P.; Moynet, D.; Boniface, K.; Richard, E.; Krisa, S.; Rambert, J.; Merillon, J.M.; et al. Malvidin-3-O-beta glucoside, major grape anthocyanin, inhibits human macrophage-derived inflammatory mediators and decreases clinical scores in arthritic rats. Biochem. Pharmacol. 2013, 86, 1461–1467. [Google Scholar] [CrossRef]
- Tsubaki, M.; Takeda, T.; Kino, T.; Itoh, T.; Imano, M.; Tanabe, G.; Muraoka, O.; Satou, T.; Nishida, S. Mangiferin suppresses CIA by suppressing the expression of TNF-alpha, IL-6, IL-1beta, and RANKL through inhibiting the activation of NF-kappaB and ERK1/2. Am. J. Transl. Res. 2015, 7, 1371–1381. [Google Scholar] [PubMed]
- Sultana, F.; Neog, M.K.; Rasool, M. Targeted delivery of morin, a dietary bioflavanol encapsulated mannosylated liposomes to the macrophages of adjuvant-induced arthritis rats inhibits inflammatory immune response and osteoclastogenesis. Eur. J. Pharm. Biopharm. 2017, 115, 229–242. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, J.; Wei, T.; Gao, J.; He, H.; Chang, X.; Yan, T. Effects of Naringenin on inflammation in complete freund’s adjuvant-induced arthritis by regulating Bax/Bcl-2 balance. Inflammation 2015, 38, 245–251. [Google Scholar] [CrossRef]
- Umar, S.; Hedaya, O.; Singh, A.K.; Ahmed, S. Thymoquinone inhibits TNF-alpha-induced inflammation and cell adhesion in rheumatoid arthritis synovial fibroblasts by ASK1 regulation. Toxicol. Appl. Pharmacol. 2015, 287, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Tekeoglu, I.; Dogan, A.; Ediz, L.; Budancamanak, M.; Demirel, A. Effects of thymoquinone (volatile oil of black cumin) on rheumatoid arthritis in rat models. Phytother. Res. 2007, 21, 895–897. [Google Scholar] [CrossRef]
- Vaillancourt, F.; Silva, P.; Shi, Q.; Fahmi, H.; Fernandes, J.C.; Benderdour, M. Elucidation of molecular mechanisms underlying the protective effects of thymoquinone against rheumatoid arthritis. J. Cell Biochem. 2011, 112, 107–117. [Google Scholar] [CrossRef]
- Rosillo, M.A.; Alcaraz, M.J.; Sanchez-Hidalgo, M.; Fernandez-Bolanos, J.G.; Alarcon-de-la-Lastra, C.; Ferrandiz, M.L. Anti-inflammatory and joint protective effects of extra-virgin olive-oil polyphenol extract in experimental arthritis. J. Nutr. Biochem. 2014, 25, 1275–1281. [Google Scholar] [CrossRef]
- Rosillo, M.A.; Sanchez-Hidalgo, M.; Gonzalez-Benjumea, A.; Fernandez-Bolanos, J.G.; Lubberts, E.; Alarcon-de-la-Lastra, C. Preventive effects of dietary hydroxytyrosol acetate, an extra virgin olive oil polyphenol in murine collagen-induced arthritis. Mol. Nutr. Food Res. 2015, 59, 2537–2546. [Google Scholar] [CrossRef]
- Ramadan, G.; Al-Kahtani, M.A.; El-Sayed, W.M. Anti-inflammatory and anti-oxidant properties of Curcuma longa (turmeric) versus Zingiber officinale (ginger) rhizomes in rat adjuvant-induced arthritis. Inflammation 2011, 34, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Kloesch, B.; Becker, T.; Dietersdorfer, E.; Kiener, H.; Steiner, G. Anti-inflammatory and apoptotic effects of the polyphenol curcumin on human fibroblast-like synoviocytes. Int. Immunopharmacol. 2013, 15, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Moon, D.O.; Choi, I.W.; Choi, B.T.; Nam, T.J.; Rhu, C.H.; Kwon, T.K.; Lee, W.H.; Kim, G.Y.; Choi, Y.H. Curcumin induces apoptosis and inhibits prostaglandin E(2) production in synovial fibroblasts of patients with rheumatoid arthritis. Int. J. Mol. Med. 2007, 20, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Sun, Y.; Liu, Z.; Zhang, M.; Li, C.; Cai, H. The effect of curcumin and its nanoformulation on adjuvant-induced arthritis in rats. Drug Des. Dev. Ther. 2015, 9, 4931–4942. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.K.; Noh, E.M.; Moon, S.J.; Kim, J.M.; Kwon, K.B.; Park, B.H.; You, Y.O.; Hwang, B.M.; Kim, H.J.; Kim, B.S.; et al. Emodin suppresses inflammatory responses and joint destruction in collagen-induced arthritic mice. Rheumatology 2013, 52, 1583–1591. [Google Scholar] [CrossRef]
- Zhu, X.; Zeng, K.; Qiu, Y.; Yan, F.; Lin, C. Therapeutic effect of emodin on collagen-induced arthritis in mice. Inflammation 2013, 36, 1253–1259. [Google Scholar] [CrossRef]
- Ha, M.K.; Song, Y.H.; Jeong, S.J.; Lee, H.J.; Jung, J.H.; Kim, B.; Song, H.S.; Huh, J.E.; Kim, S.H. Emodin inhibits proinflammatory responses and inactivates histone deacetylase 1 in hypoxic rheumatoid synoviocytes. Biol. Pharm. Bull. 2011, 34, 1432–1437. [Google Scholar] [CrossRef]
- Fleury, G.; Mania, S.; Hannouche, D.; Gabay, C. The perioperative use of synthetic and biological disease-modifying antirheumatic drugs in patients with rheumatoid arthritis. Swiss Med. Wkly. 2017, 147, w14563. [Google Scholar] [CrossRef]
- Cho, S.-K.; Bae, S.-C. Pharmacologic treatment of rheumatoid arthritis. J. Korean Med. Assoc. 2017, 60. [Google Scholar] [CrossRef]
- Soto, M.; Falqué, E.; Domínguez, H. Relevance of Natural Phenolics from Grape and Derivative Products in the Formulation of Cosmetics. Cosmetics 2015, 2, 259–276. [Google Scholar] [CrossRef]


| Compound | Source | Cell Line/Animal Model | Dose/Duration | Mechanism | Reference |
|---|---|---|---|---|---|
| Ferulic acid | Grains (rice, wheat and oats), vegetables, fruits, nuts | monocyte/macrophage cells/Rat | 25, 50, 100 μM/24 h | ↓ NFATc1, c-Fos, NF-κB, TRAP, MMP-9, Cathepsin | [10] |
| Natural polyphenol N-feruloylserotonin (N-f-5HT) | Leuzea carthamoides | AA | 3 mg/kg/28 days | ↓ CRP, LOX, TNF-α, iNOS, IL-1β | [11] |
| Gallotanins | Euphorbia | HMC-1/human | 10 mg/mL/30 min | ↓ TNF- α, IL-1β, IL-6, NF-κB | [12] |
| Kaempferol (3,5,7,4′-tetrahydroxy-flavone) | Gallic acid | RASFs/human | 100 µM/ 2 days | ↓ IL-1β, MMPs, COX, PGE2 | [16] |
| Chlorogenic acid (CGA) | Gardenia jasminoides | osteoclast/ BMMs | 10, 25, 50 μg/mM/4 days | ↓ NF-κB, P38, Akt, ERK | [13] |
| p-Coumaric Acid (CA) | Gnetm cleistostachyum | AIA | 100 mg/kg/8 days | ↓TNF-α, CIC ↑ IgG | [14] |
| p-Coumaric Acid (CA) | Gnetm cleistostachyum | AIA | 100 mg/kg/16 days | ↓ TNF-α, IL-1β, IL-6, MCP-1, RANKL, TRAP, IL-1β, IL-6, IL-17, iNOS, COX-2, NF-κB-p65, p-NF-κB-p65, NFATc-1, c-Fos, JNK, p-JNK, ERK1/2 ↑OPG | [15] |
| Compound | Source | Cell Line/Animal Model | Dose/Duration | Mechanism | Reference |
|---|---|---|---|---|---|
| Resveratrol | Red grapes | FLSs/AA | 5, 15, 45 mg/kg/12 days | ↑ MtROS ↓ Beclin1, LC3A/B, MnSOD | [18] |
| Resveratrol | Red grapes | FLSs/Human | 50 μg/24 h | ↓ COX-2, PGE2, NADPH oxidase, ROS, Akt, p38, MAPK, ERK1/2, NF-κB | [19] |
| Resveratrol | Red grapes | FLSs/Human | 6.25, 12.5, 25, 50 µM/1 h | ↓ IL-1β, MMP-3, P-Akt, PI3K-Akt | [20] |
| Resveratrol | Red grapes | Human * randomized controlled clinical trial | 1000 mg/day/3 month | ↓ RF, MMP-3, TNF-α, IL-6, | [21] |
| Resveratrol | Red grapes |
|
|
| [22] |
| Resveratrol | Red grapes | CFA induced rat | 10 mg/kg/day/7 days | RF, MMP-3, COMP, IgG, ANA, TNF-a, MPO, MDA ↑ IL-10, GSH | [23] |
| Compound | Source | Cell Line/Animal Model | Dose/Duration | Mechanism | Reference |
|---|---|---|---|---|---|
| A-glucosylhesperidin | Citrus fruit | CIA rat | 3 mg/0.3 mL/3 times a week, 31 days | ↓ TNFα | [27] |
| Anthocyanin | Cherries | AIA rat (Male Sprague Dawley) | 10, 20, 40 mg/kg/14 days | ↓TNFα, PGE2, MDA ↑ SOD | [28] |
| Cocoa polyphenol (epicatechin, catechins, flavonol glycosides and procyanidin) | Cocoa | JB6 P+ mouse epidermal cells | 0, 10, 20 μM /mL/1 h | ↓ VEGF, NF-kB, AP-1 ↓ p-Akt, p-p70S6K, p- ERK, p- p90RSK, p- MKK4, p-JNK, p- PI3K | [29] |
| Epigallocatechin-3-gallate (EGCG) | Green tea (Camellia sinensis) | RASFs | 10, 20, 30, 40, 50 μM/12 h | ↓ENA-78, RANTES, GRO-alpha, MMP-2 | [30] |
| Epigallocatechin-3-gallate (EGCG) | Green tea | CIA rat (DBA/1J) | 20, 30, 40, 50 mg/kg/3 weeks | ↓ IgG2a, IL-1β, IL-6, TNFα, TRAP, IL-17, VEGF, nitrotyrosine, iNOS, p-STAT3, c-Fos, NFATc1, CTSK, MMP9, p-STAT3 727, IL-17, CCL6, AHR, IL-21, p-STAT3 705, p-ERK, RANK, CTR ↑ IL-10, TGF- β, SOCS3, Foxp3 | [32] |
| Epigallocatechin gallate | Green tea | PIA rats (Dark Agouti) | 10 mg/kg/5 days | ↓ MPO | [34] |
| Epigallocatechin-3-gallate (EGCG) | Green tea (Camellia sinensis) |
| 20 μM, 50 μM/15 days | ↓ CTR, carbonic anhydrase II, cathepsin K, alpha-v integrin, β-3 integrin, NF-ATc1 | [35] |
| Epigallocatechin-3-gallate (EGCG) | Green tea | CIA rat (DBA/1J) | 10 mg/kg/3 weeks | ↓ IL-6, TNFα, IFN-γ ↑anti-CII specific IgG1 antibodies | [33] |
| Epigallocatechin-3-gallate (EGCG) | Camellia sinensis | Osteoclast precursors cells mature osteoclasts | 10, 100 μM/7 days | ↓ Multinucleated osteoclast formation, MMP-9, MMP-2 | [36] |
| Epigallocatechin 3-gallate (EGCG) | Green tea (Camellia sinensis) | RASFs | 125, 250, 500 nM/24 h | ↓ MAPK, MMP-1, MMP-3, p-ERK1/2, p-JNK, p-p38, AP-1 | [31] |
| Fisetin | Rhus verniciflua Stokes | RA FLs | 0.1, 1, 10 μg/mL/72 h | ↓ TNFα, IL-6, IL-8, MCP-1, VEGF | [37] |
| Flavonol-rich residual layer of hexane fraction (RVHxR) | Rhus verniciflua Stokes | RA FLs | 0.1, 1, 10 μg/mL/72 h | ↓ TNFα, IL-6, IL-8, MCP-1, VEGF ↓ p-ERK, p-JNK, ↑ p- p38-MAPK | [37] |
| Gallic acid | Cinnamomum zeylanicum Bark |
|
|
| [38] |
| Genistein | CIA rats | 1 mL/kg/42 days | ↓ IFN-γ, Th1/Th2, T-bet ↑ GATA-3, IL-4 | [39] | |
| Genistein | Soybean | RA FLS | 10 μg/mL/24 h | ↓ MMP-9 | [40] |
| Hesperidin | CIA rat (Wistar rat) | 160 mg/kg / 22 days | ↓ ELA, TBARS, nitrite ↑ GSH, SOD, catalase | [41] | |
| Malvidin-3-O-β-glucoside | Red grape skinExtract powder |
|
|
| [42] |
| Mangiferin | Thymelaeaceae family (e.g., Phaleria cumingii) | CIA rat (DBA/1) | 100 and 400 mg/kg/14 days and 27 days | ↓ NF-κB, ERK1/2,IL-1β, IL-6, TNF-α, RANKL | [43] |
| Morin (ML-morin) | Fruits, vegetables, tea | Spleen and synovial macrophages | 10 mg/kg/3 days | ↓ ROS, NO, iNOS, NF-κB-p65, TNF-α, IL-1 β, IL-6, MCP-1, VEGF, RANKL, STAT-3 | [44] |
| Naringin | Grape, citrus fruit | AIA rat (Female Sprague-Dawley) |
| ↓ TNFα, IL-1β, IL-6, Bcl-2 ↑ Bax | [45] |
| Theaflavin-3,3′-digallate (TFDG) | Camellia sinensis | osteoclast precursors cells mature osteoclasts | 10, 100 μM/7 days | ↓ Multinucleated osteoclast formation, MMP-9, MMP-2 | [36] |
| Thymoquinone | Nigella sativa | RA synovium | 1, 2, 3, 4, 5 μM/2 h | ↓ IL-6, IL-8, ICAM-1, VCAM-1, Cad-11, p38, JNK | [46] |
| Thymoquinone | Nigella sativa | CIA rat (Sprague-Dawley Wistar rat) | 2.5 mg/kg/5 days 5 mg/kg/5 days | ↓ IL-1β | [47] |
| Thymoquinone | Nigella sativa |
|
|
| [48] |
| Compound | Source | Cell Line/Animal Model | Dose/Duration | Mechanism | Reference |
|---|---|---|---|---|---|
| EVOO-polyphenol extract (PE) | EVOO | CIA in DBA-1/J | 100, 200 mg/kg/13 days | ↓ TNF-α, IL-1β, IL-6, PEG2, p38, JNK, p65, lκB- α | [49] |
| Hydroxytyrosol acetate (Hty-Ac) | EVOO | CIA in DBA-1/J | 0.05%/42 days | ↓ IgG1, IgG2a, COMP, MMP-3, TNF-Q, IFN-S, IL-1R, IL-6, IL-17A, Nrf2, HO-1 | [50] |
| Curcuminoid |
| AIA | 200 mg/kg/28 days | ↑ TNF-α, IL-1β, IL-6, IL-4, IL-10, SOD, CAT, GSH ↓ LPO, ALAT, ALP | [51] |
| Curcumin | Turmeric rhizome |
| 12.5, 25, 50 μM/6 h |
| [52] |
| Curcumin oil-water nanoemulsions (CM-Ns) | Herb turmeric | AIA | 50 mg/kg/14 days | ↓ NF-κB, TNF-α, IL-1β | [54] |
| Curcumin | Rhizome of Curcuma longa | FLS/Patient | 0, 25, 50, 75, 100 μM/24 h | ↓ Bcl-2, COX-2↑caspase-3, caspase-9 | [53] |
| Emodin | Rheum palmatum | CIA DBA/1 J | 10 mg/kg/11 days | ↓ NF-κB, MMP, M-CSF | [55] |
| Emodin | Rheum palmatum | CIA | 5, 10, 20 mg/kg/21 days | ↓ TNF-α, IL-6, PGE2 | [56] |
| Emodin | Rheum palmatum | Synovial membrane/Humans | 0.1, 1, 10 μM/24 h | ↓ HDAC, HDAC1, VEGF, COX-2, HIF-1a, MMP-1, MMP-13, NF-κB, MAPK | [57] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sung, S.; Kwon, D.; Um, E.; Kim, B. Could Polyphenols Help in the Control of Rheumatoid Arthritis? Molecules 2019, 24, 1589. https://doi.org/10.3390/molecules24081589
Sung S, Kwon D, Um E, Kim B. Could Polyphenols Help in the Control of Rheumatoid Arthritis? Molecules. 2019; 24(8):1589. https://doi.org/10.3390/molecules24081589
Chicago/Turabian StyleSung, Siyun, Doyoung Kwon, Eunsik Um, and Bonglee Kim. 2019. "Could Polyphenols Help in the Control of Rheumatoid Arthritis?" Molecules 24, no. 8: 1589. https://doi.org/10.3390/molecules24081589
APA StyleSung, S., Kwon, D., Um, E., & Kim, B. (2019). Could Polyphenols Help in the Control of Rheumatoid Arthritis? Molecules, 24(8), 1589. https://doi.org/10.3390/molecules24081589