Concentration of EPA and DHA from Refined Salmon Oil by Optimizing the Urea–Fatty Acid Adduction Reaction Conditions Using Response Surface Methodology
Abstract
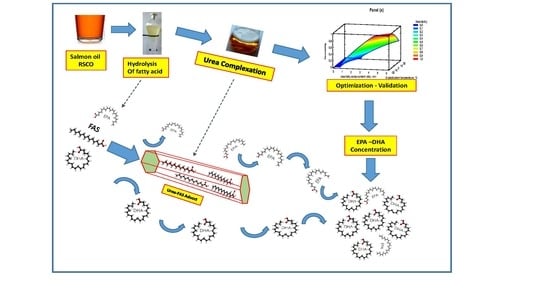
:1. Introduction
2. Results
2.1. Characterization of the Initial Refined Commercial Salmon Oil
2.2. Effect of Process Variables on Total FA Yield, EPA Contents, and DHA Contents of RCSO Concentrate
2.2.1. Refined Commercial Salmon Oil Concentrate
2.2.2. Effect of Process Variables on EPA, DHA, and EPA+DHA Content and Total FA Yield: Pareto Charts and RSM Analysis
2.2.3. Models Obtained for the Concentration of EPA, DHA, and EPA+DHA
2.2.4. Independent Variables and Multiple Response Optimization
2.2.5. Validation of the Optimized Process and Characterization of the EPA+DHA Content Obtained
2.3. Composition of FA in the RCSO Optimized Concentrate after Validation
3. Discussion
4. Materials and Methods
4.1. Materials and Chemicals
4.2. Characterization of Refined Commercial Salmon Oil
4.3. FA Composition of the RCSO and n-3 LCPUFA Concentrates
4.4. n-3 LCPUFA Concentrates from RCSO
4.5. Experimental Design and Optimization Procedure
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Food and Agriculture Organization/World Health Organization (FAO/WHO). Fats and Fatty Acids in Human Nutrition; Report of an Expert Consultation; FAO/WHO: Rome, Italy, 2010; 166p, ISBN 978-92-5-106733-8. [Google Scholar]
- Schuncka, W.; Konkelb, A.; Fischerb, R.; Weylandtc, K. Therapeutic potential of omega-3 fatty acid-derived epoxyeicosanoids in cardiovascular and inflammatory diseases. Pharm. Therap. 2018, 183, 177–204. [Google Scholar] [CrossRef] [PubMed]
- Siscovick, D.S.; Barringer, T.A.; Fretts, A.M.; Wu, J.H.Y.; Lichtenstein, A.H.; Costello, R.B.; Kris-Etherton, P.M.; Jacobson, T.A.; Engler, M.B.; Alger, H.M.; et al. Omega-3 polyunsaturated fatty acid (fish oil) supplementation and the prevention of clinical cardiovascular disease: A science advisory from the American Heart Association. American Heart Association Nutrition Committee of the Council on Lifestyle and Cardiometabolic Health; Council on Epidemiology and Prevention; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing and Council on Clinical Cardiology. Circulation 2017, 135, e867–e884. [Google Scholar]
- Weylandt, K.; Schmöcker, C.; Ostermann, A.; Kutzner, L.; Willenberg, I.; Kiesler, S.; Steinhagen-Thiessen, E.; Schebb, N.; Kassner, U. Activation of lipid mediator formation due to lipoprotein apheresis. Nutrients 2019, 11, 363. [Google Scholar] [CrossRef]
- Swanson, S.; Block, R.; Mousa, S.A. Omega-3 fatty acids EPA and DHA: Health benefits throughout life. Adv. Nutr. 2012, 3, 1–7. [Google Scholar] [CrossRef]
- Haast, R.; Kiliaan, A. Impact of fatty acids on brain circulation, structure and function. Prost. Leuk. Ess. Fatty Ac. J. 2015, 92, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Minihane, A.; Armah, C.; Miles, E.; Madden, J.; Clark, A.; Caslake, M.; Calder, P. Consumption of fish oil providing amounts of eicosapentaenoic acid and docosahexaenoic acid that can be obtained from the diet reduces blood pressure in adults with systolic hypertension: A retrospective analysis. J. Nut. 2016, 146, 516–523. [Google Scholar] [CrossRef]
- WHO. World Health Statistics: Monitoring health for the SDGs, Sustainable Development Goals; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Ofosu, F.K.; Daliri, E.B.M.; Lee, B.H.; Yu, X. Current trends and future perspectives on omega-3 fatty acids. Res. J. Biol. 2017, 5, 11–20. [Google Scholar]
- Zanoaga, O.; Jurj, A.; Raduly, L.; Cojocneanu-Petric, R.; Fuentes-Mattei, E.; Wu, O.; Braicu, C.; Gherman, C.D.; Berindan-Neagoe, I. Implications of dietary ω-3 and ω-6 polyunsaturated fatty acids in breast cancer (Review). Experim. Therap. Med. 2018, 15, 1167–1176. [Google Scholar] [CrossRef]
- Rubio-Rodríguez, N.; Beltrán, S.; Jaime, I.; De Diego, S.M.; Sanz, M.T.; Rovira, J. Production of omega-3 polyunsaturated fatty acid concentrates: A review. Innov. Food Sci. Em. Technol. 2010, 11, 1–12. [Google Scholar] [CrossRef]
- Haq, M.; Getachew, A.T.; Saravana, P.S.; Cho, Y.J.; Park, S.K.; Kim, M.J.; Chun, B.-S. Effects of process parameters on EPA and DHA concentrate production from Atlantic salmon by-product oil: Optimization and characterization. Kor. J. Chem. Eng. 2016, 34, 2255–2264. [Google Scholar] [CrossRef]
- Wanasundara, U.; Shahidi, F. Concentration of omega 3- polyunsaturated fatty acids of seal blubber oil by urea complexation: Optimization of reaction conditions. Food Chem. 1999, 65, 41–49. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, C.; Hong, P.; Ji, H. Concentration of docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) of tuna oil by urea complexation: Optimization of process parameters. J. Food Eng. 2006, 73, 203–209. [Google Scholar] [CrossRef]
- Patil, D. Recent trends in production of polyunsaturated fatty acids (PUFA) Concentrates. J. Food Res Technol. 2014, 2, 15–23. [Google Scholar]
- Thammapat, P.; Siriamornpun, S.; Raviyan, P. Concentration of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) of Asian catfish oil by urea complexation: Optimization of reaction conditions. J. Sci. Technol. 2016, 38, 163–170. [Google Scholar]
- Pando, M.; Rodríguez, A.; Galdames, A.; Berríos, M.M.; Rivera, M.; Romero, N.; Valenzuela, M.A.; Ortiz, J.; Aubourg, S.P. Maximization of the docosahexaenoic and eicosapentaenoic acids content in concentrates obtained from a by-product of rainbow trout (Oncorhynchus mykiss) processing. Eur. Food Res. Technol. 2018, 244, 937–948. [Google Scholar] [CrossRef]
- Pando, M.; Bravo, B.; Berríos, M.M.; Galdames, A.; Rojas, C.; Romero, N.; Camilo, C.; Rodriguez, A.; Aubourg, S.P. Concentrating n-3 fatty acids from crude and refined commercial salmon oil. Czech J. Food Sci. 2014, 32, 169–176. [Google Scholar] [CrossRef]
- Roca-Saavedra, P.; Mariño-Lorenzo, P.; Miranda, J.M.; Porto-Arias, J.J.; Lamas, A.; Vazquez, B.I.; Franco, C.M.; Cepeda, A. Phytanic acid consumption and human health, risks, benefits and future trends: A review. Food Chem. 2017, 15, 237–247. [Google Scholar] [CrossRef]
- Haliloğlu, H.I.; Bayır, A.; Sirkecioğlu, A.N.; Aras, N.M.; Atamanalp, P. Comparison of fatty acid composition in some tissues of rainbow trout (Oncorhynchus mykiss) living in seawater and freshwater. Food Chem. 2004, 86, 55–59. [Google Scholar] [CrossRef]
- Guil-Guerrero, J.L.; Belarbi, H. Purification process for cod liver oil polyunsatured fatty acids. J. Am. Oil Chem. Soc. 2001, 78, 477–484. [Google Scholar] [CrossRef]
- Fei, Ch.; Salimon, J.; Said, M. Optimization of Urea Complexation by Box-Behnken Design. Sains Malaysiana. 2010, 39, 795–803. [Google Scholar]
- De Boer, A.; Ismail, A.; Marshall, K.; Bannenberg, G.; Yan, K.L.; Rowe, W.J. Examination of marine and vegetable oil oxidation data from a multi-year, third-party database. Food Chem. 2018, 254, 249–255. [Google Scholar] [CrossRef]
- Méndez, C.; Masson, L.; Jiménez, P. Estabilización de aceite de pescado por medio de antioxidantes naturales. Aceites y Grasas 2010, 80, 492–500. [Google Scholar]
- AOAC. Official Methods and Recommended Practices of the American Oil Chemists Society (edited by AOCS); AOAC: Champaign, IL, USA, 1993. [Google Scholar]
- AOCS. Determination of Cis-, Trans-, Saturated, Monounsaturated, and Polyunsaturated Fatty Acids by Capillary Gas Liquid Chromatography (GLC). Official Method Ce 1j-7. Official Methods and Recommended Practices of the American Oil Chemists Society (edited by AOCS); AOCS: Champaign, IL, USA, 2009. [Google Scholar]
- Tran, Q.T.; Le, T.T.T.; Pham, M.Q.; Do, T.L.; Vu, M.H.; Nguyen, D.C.; Bach, L.G.; Bui, L.M.; Pham, Q.L. Fatty acid, lipid classes and phospholipid molecular species composition of the marine Clam Meretrix lyrata (Sowerby) from Cua Lo Beach, Nghe An Province, Vietnam. Molecules 2019, 24, 895. [Google Scholar] [CrossRef]
- Kieliszek, M.; Błażejak, S.; Bzducha-Wróbel, A.; Kot, A.M. Effect of selenium on lipid and amino acid metabolism in yeast cells. Biol. Trace Elem. Res. 2019, 187, 316–327. [Google Scholar] [CrossRef]
- Berríos, M.; Rodríguez, A.; Rivera, M.; Pando, M.; Valenzuela, M.A.; Aubourg, S.P. Optimization of rancidity stability in long-chain PUFA concentrates obtained from a rainbow trout (Oncorhynchus mykiss) by-product. Int. J. Food Sci. Technol. 2017, 52, 1463–1472. [Google Scholar] [CrossRef]
- Zuta, C.P.; Simpson, B.K.; Chan, H.M.; Phillips, L. Concentrating PUFA from Mackerel Processing Waste. J. AOCS. 2003, 80, 933–936. [Google Scholar] [CrossRef]
- Myers, R.H.; Montgomery, D.C. Response Surface Methodology: Process and Product Optimization Using Design Experiments; John Wiley & Sons, Inc.: New York, NY, USA, 1995. [Google Scholar]
- Derringer, G.; Suich, R. Simultaneous Optimization of Several Response Variables. J. Qual. Technol. 1980, 12, 214–219. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |


| FA or FA Groups | RCSO | RCSO Optimum |
|---|---|---|
| 12:0 | 0.07 | Nd |
| 14:0 | 3.19 | 0.12 |
| 15:0 | 0.20 | 0.09 |
| 16:0 | 13.74 | Nd |
| 16:1 9t | 0.15 | Nd |
| 16:1 7c | Nd | Nd |
| 16:1 9c | 4.66 | 0.51 |
| 16:1 11c | Nd | Nd |
| 16:1 13c | Nd | Nd |
| 17:0 | 0.13 | 0.10 |
| 17:1 10c | 0.56 | 1.28 |
| 18:0 | 3.69 | 0.28 |
| 18:1 9c | 29.61 | 0.59 |
| 18:1 11c | 3.69 | 0.04 |
| 18:2 9t, 12t | Nd | Nd |
| 18:2 9c, 12c | 16.69 | 7.48 |
| 18:2 9c, 15c | Nd | Nd |
| 18:3 6c, 9c, 12c | 0.22 | 1.09 |
| 20:0 | 0.26 | Nd |
| 18:3 9c, 12c, 15c | 3.25 | 2.60 |
| 20:1 5c | Nd | Nd |
| 20:1 8c | Nd | Nd |
| 20:1 11c | 1.60 | Nd |
| 18:4 6c, 9c, 12c, 15c | Nd | Nd |
| 20:2 11c, 14c | 0.79 | 0.05 |
| 20:3 8c, 11c, 14c | 0.30 | 1.16 |
| 20:3 11c, 14c, 17c | 0.12 | 0.03 |
| 20:4 8c, 11c, 14c, 17c | 0.40 | 1.37 |
| 22:1 13c | 0.21 | Nd |
| 20:5 5c, 8c, 11c, 14c, 17c | 7.53 | 31.20 |
| 24:1 15c | Nd | Nd |
| 22:5 7c, 10c, 13c, 16c, 19c | 2.69 | 2.70 |
| 22:6 4c, 7c, 10c, 13c, 16c, 19c | 6.25 | 49.31 |
| Total SFAs | 21.28 | 0.59 |
| Total MUFAs | 40.48 | 2.42 |
| Total PUFAs | 38.24 | 96.99 |
| Total n-3PUFAs | 20.45 | 87.21 |
| Total n-3LCPUFAs | 18.08 | 85.82 |
| EPA+DHA | 13.78 | 80.51 |
| Run | Process Variables * | Response Variables ** | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Experimental Values | Predicted Values | |||||||||
| A | B | C | D | R1 | R2 | R3 | R1’ | R2’ | R3’ | |
| 1 | 1.5 | −15 | 14.3 | 200 | 40.44 | 10.47 | 11.04 | 33.77 | 13.77 | 14.00 |
| 2 | 4.5 | −15 | 14.3 | 200 | 12.64 | 28.41 | 44.38 | 12.23 | 28.09 | 45.89 |
| 3 | 1.5 | 15 | 14.3 | 200 | 48.71 | 9.22 | 9.52 | 40.35 | 9.52 | 10.23 |
| 4 | 4.5 | 15 | 14.3 | 200 | 17.45 | 25.09 | 32.69 | 18.82 | 23.85 | 26.94 |
| 5 | 1.5 | −15 | 36.8 | 200 | 36.25 | 10.46 | 11.13 | 29.02 | 13.22 | 15.24 |
| 6 | 4.5 | −15 | 36.8 | 200 | 10.44 | 24.42 | 55.91 | 21.55 | 27.55 | 47.14 |
| 7 | 1.5 | 15 | 36.8 | 200 | 4.22 | 7.96 | 8.18 | 20.22 | 8.98 | 11.47 |
| 8 | 4.5 | 15 | 36.8 | 200 | 18.12 | 24.87 | 30.47 | 12.76 | 23.30 | 28.18 |
| 9 | 1.5 | −15 | 14.3 | 600 | 41.74 | 10.23 | 10.89 | 44.89 | 14.52 | 14.71 |
| 10 | 4.5 | −15 | 14.3 | 600 | 12.77 | 30.20 | 46.43 | 12.14 | 28.84 | 46.61 |
| 11 | 1.5 | 15 | 14.3 | 600 | 78.45 | 9.02 | 9.41 | 68.43 | 10.27 | 10.94 |
| 12 | 4.5 | 15 | 14.3 | 600 | 16.11 | 25.16 | 34.34 | 21.40 | 24.60 | 27.65 |
| 13 | 1.5 | −15 | 36.8 | 600 | 45.49 | 9.91 | 10.06 | 40.14 | 13.97 | 15.96 |
| 14 | 4.5 | −15 | 36.8 | 600 | 9.86 | 27.56 | 48.55 | 7.17 | 28.30 | 47.85 |
| 15 | 1.5 | 15 | 36.8 | 600 | 58.94 | 7.84 | 7.91 | 48.30 | 9.73 | 12.18 |
| 16 | 4.5 | 15 | 36.8 | 600 | 12.64 | 23.05 | 28.93 | 15.33 | 24.05 | 28.89 |
| 17 | 0 | 0 | 25.5 | 400 | 57.38 | 6.33 | 6.39 | 67.84 | -0.77 | 0.94 |
| 18 | 6 | 0 | 25.5 | 400 | 15.86 | 25.45 | 30.43 | 13.34 | 27.88 | 49.55 |
| 19 | 3 | −30 | 25.5 | 400 | 12.21 | 29.26 | 44.37 | 19.61 | 24.94 | 36.61 |
| 20 | 3 | 30 | 25.5 | 400 | 33.82 | 13.52 | 14.65 | 34.36 | 16.45 | 13.88 |
| 21 | 3 | 0 | 3.05 | 400 | 12.71 | 20.41 | 22.67 | 25.65 | 21.24 | 24.00 |
| 22 | 3 | 0 | 48.0 | 400 | 16.31 | 23.01 | 28.93 | 14.83 | 20.15 | 26.49 |
| 23 | 3 | 0 | 25.5 | 0 | 19.11 | 22.62 | 25.72 | 13.39 | 19.95 | 24.53 |
| 24 | 3 | 0 | 25.5 | 800 | 16.47 | 26.07 | 33.39 | 27.09 | 21.44 | 25.95 |
| 25 | 3 | 0 | 25.5 | 400 | 20.77 | 22.43 | 25.82 | 20.24 | 20.69 | 25.24 |
| 26 | 3 | 0 | 25.5 | 400 | 20.74 | 21.40 | 25.20 | 20.24 | 20.69 | 25.24 |
| 27 | 3 | 0 | 25.5 | 400 | 17.20 | 24.00 | 29.15 | 20.24 | 20.69 | 25.24 |
| 28 | 3 | 0 | 25.5 | 400 | 22.74 | 18.23 | 20.23 | 20.24 | 20.69 | 25.24 |
| Equations | R2 Adjusted | |||||||||
| Total FA yield = 57.60 − 19.47A + 0.26B − 0.87C + 0.08D + 2.26AA+ 0.01BB + 0.21AC − 0.02AD − 0.02BC + 0.001BD | 0.72(1) | |||||||||
| EPA = −0.90 + 9.53A − 0.14B − 0.79AA | 0.84(2) | |||||||||
| DHA = −1.19 + 8.10A + 0.17B − 0.19AB | 0.81(3) | |||||||||
| EPA+DHA = 3.52 + 12.88A − 0.52B | 0.80(4) | |||||||||
| Part a) Optimization of the Process Variables | ||||||
| Dependent Variables | Process Variables | Stationary Point | Optimum Value ** | |||
| A | B | C | D | |||
| EPA | 5.99 | −29.79 | 3.05 | 599.00 | Maximum | 33.01 |
| DHA | 6.00 | −29.98 | 48.05 | 108.30 | Maximum | 76.81 |
| EPA+DHA | 6.00 | −29.95 | 47.79 | 271.36 | Maximum | 98.85 |
| Part b) Multiple Response Optimization of the Response Variables | ||||||
| Dependent Variables | Process Variables | Stationary Point | Predicted Value ** | |||
| A | B | C | D | |||
| EPA | 5.84 | −17.69 | 14.83 | 453.36 | Maximum | 30.71- |
| DHA | 62.94 | |||||
| EPA+DHA | 90.07 | |||||
| Maximum desirability | 1.0 | |||||
| Part c) Experimental Validation of the Multiple Response Optimization of the Dependent Variables | ||||||
| Dependent Variables | Process Variables | Stationary Point | Experimental Value ** | |||
| A | B | C | D | |||
| EPA | 6.00 | −18 | 14.80 | 500 | Maximum | 31.20 |
| DHA | 49.31 | |||||
| EPA+DHA | 80.51 | |||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dovale-Rosabal, G.; Rodríguez, A.; Contreras, E.; Ortiz-Viedma, J.; Muñoz, M.; Trigo, M.; Aubourg, S.P.; Espinosa, A. Concentration of EPA and DHA from Refined Salmon Oil by Optimizing the Urea–Fatty Acid Adduction Reaction Conditions Using Response Surface Methodology. Molecules 2019, 24, 1642. https://doi.org/10.3390/molecules24091642
Dovale-Rosabal G, Rodríguez A, Contreras E, Ortiz-Viedma J, Muñoz M, Trigo M, Aubourg SP, Espinosa A. Concentration of EPA and DHA from Refined Salmon Oil by Optimizing the Urea–Fatty Acid Adduction Reaction Conditions Using Response Surface Methodology. Molecules. 2019; 24(9):1642. https://doi.org/10.3390/molecules24091642
Chicago/Turabian StyleDovale-Rosabal, Gretel, Alicia Rodríguez, Elyzabeth Contreras, Jaime Ortiz-Viedma, Marlys Muñoz, Marcos Trigo, Santiago P. Aubourg, and Alejandra Espinosa. 2019. "Concentration of EPA and DHA from Refined Salmon Oil by Optimizing the Urea–Fatty Acid Adduction Reaction Conditions Using Response Surface Methodology" Molecules 24, no. 9: 1642. https://doi.org/10.3390/molecules24091642
APA StyleDovale-Rosabal, G., Rodríguez, A., Contreras, E., Ortiz-Viedma, J., Muñoz, M., Trigo, M., Aubourg, S. P., & Espinosa, A. (2019). Concentration of EPA and DHA from Refined Salmon Oil by Optimizing the Urea–Fatty Acid Adduction Reaction Conditions Using Response Surface Methodology. Molecules, 24(9), 1642. https://doi.org/10.3390/molecules24091642