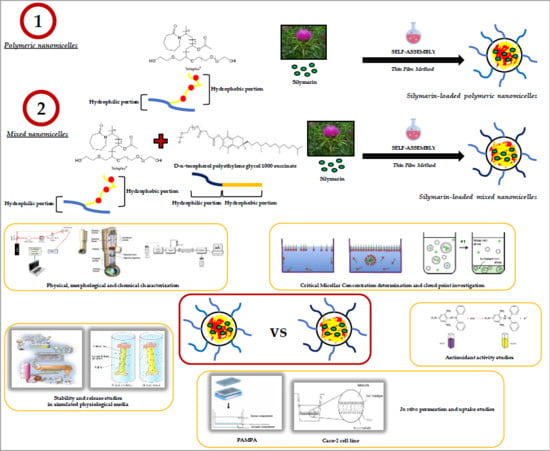
Formulation of Nanomicelles to Improve the Solubility and the Oral Absorption of Silymarin
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation and Characterization of Nanomicelles
2.2. Solubilization Capacity Determination
2.3. Cloud Point
2.4. Stability Studies
2.4.1. Storage Stability
2.4.2. Gastrointestinal Stability
2.4.3. Stability in Blood Conditions
2.5. In Vitro Release Studies
2.6. Parallel Artificial Membrane Permeability Assay (PAMPA)
2.7. Caco-2 Experiments
2.8. DPPH Assay
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Nanomicelles Fabrication
3.2.2. Theoretical Critical Micellar Concentration (CMC)
3.2.3. Determination of Solubilization Capacity
3.2.4. Nanomicelles’ Physical and Morphological Characterization
3.2.5. Drug Loading and Encapsulation Efficiency
3.2.6. Cloud Point
3.2.7. Stability Studies
Storage Stability Studies
Gastrointestinal Stability Studies
Stability in Blood Conditions
3.2.8. In Vitro Release Studies
3.2.9. PAMPA Studies
3.2.10. Caco-2 Cell Lines
Transport Studies
Uptake Studies
RT-PCR
3.2.11. Antioxidant Activity Studies
3.2.12. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chambers, C.S.; Holečková, V.; Petrásková, L.; Biedermann, D.; Valentová, K.; Buchta, M.; Křen, V. The silymarin composition and why does it matter? Food Res. Int. 2017, 100, 339–353. [Google Scholar] [CrossRef]
- Martinelli, T.; Whittaker, A.; Benedettelli, S.; Carboni, A.; Andrzejewska, J. The study of flavonolignan association patterns in fruits of diverging Silybum marianum (L.) Gaertn. chemotypes provides new insights into the silymarin biosynthetic pathway. Phytochemistry 2017, 144, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Bijak, M. Silybin, a Major Bioactive Component of Milk Thistle (Silybum marianum L. Gaernt.)—Chemistry, Bioavailability, and Metabolism. Molecules 2017, 22, 1942. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimpour-Koujan, S.; Gargari, B.P.; Mobasseri, M.; Valizadeh, H.; Asghari-Jafarabadi, M. Lower glycemic indices and lipid profile among type 2 diabetes mellitus patients who received novel dose of Silybum marianum (L.) Gaertn. (silymarin) extract supplement: A Triple-blinded randomized controlled clinical trial. Phytomedicine 2018, 44, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Manna, S.K.; Mukhopadhyay, A.; Van, N.T.; Aggarwal, B.B. Silymarin suppresses TNF-induced activation of NF-κB, c-Jun N-terminal kinase, and apoptosis. J. Immunol. 1999, 163, 6800–6809. [Google Scholar] [PubMed]
- Sayyah, M.; Boostani, H.; Pakseresht, S.; Malayeri, A. Comparison of Silybum marianum (L.) Gaertn. with fluoxetine in the treatment of Obsessive−Compulsive Disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 2010, 34, 362–365. [Google Scholar] [CrossRef]
- Zholobenko, A.; Modriansky, M. Silymarin and its constituents in cardiac preconditioning. Fitoterapia 2014, 97, 122–132. [Google Scholar] [CrossRef] [Green Version]
- Soto, C.; Mena, R.; Luna, J.; Cerbón, M.; Larrieta, E.; Vital, P.; Uría, E.; Sánchez, M.; Recoba, R.; Barrón, H.; et al. Silymarin induces recovery of pancreatic function after alloxan damage in rats. Life Sci. 2004, 75, 2167–2180. [Google Scholar] [CrossRef]
- Breschi, M.; Martinotti, E.; Apostoliti, F.; Nieri, P. Protective effect of silymarin in antigen challenge- and histamine-induced bronchoconstriction in in vivo guinea-pigs. Eur. J. Pharmacol. 2002, 437, 91–95. [Google Scholar] [CrossRef]
- Khan, A.Q.; Khan, R.; Tahir, M.; Rehman, M.U.; Lateef, A.; Ali, F.; Hamiza, O.O.; Hasan, S.K.; Sultana, S. Silibinin Inhibits Tumor Promotional Triggers and Tumorigenesis Against Chemically Induced Two-Stage Skin Carcinogenesis in Swiss Albino Mice: Possible Role of Oxidative Stress and Inflammation. Nutr. Cancer 2013, 66, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Soto, C.; Pérez, J.; García, V.; Uría, E.; Vadillo, M.; Raya, L. Effect of silymarin on kidneys of rats suffering from alloxan-induced diabetes mellitus. Phytomedicine 2010, 17, 1090–1094. [Google Scholar] [CrossRef]
- Elyasi, S.; Hosseini, S.; Moghadam, M.R.N.; Aledavood, S.A.; Karimi, G. Effect of Oral Silymarin Administration on Prevention of Radiotherapy Induced Mucositis: A Randomized, Double-Blinded, Placebo-Controlled Clinical Trial. Phytother. Res. 2016, 30, 1879–1885. [Google Scholar] [CrossRef]
- Yap, Y.S.; Kwok, L.L.; Syn, N.; Chay, W.Y.; Chia, J.W.K.; Tham, C.K.; Wong, N.S.; Lo, S.K.; Dent, R.A.; Tan, S.; et al. Predictors of Hand-Foot Syndrome and Pyridoxine for Prevention of Capecitabine–Induced Hand-Foot Syndrome: A Randomized Clinical Trial. JAMA Oncol. 2017, 3, 1538–1545. [Google Scholar] [CrossRef]
- Dixit, N.; Baboota, S.; Kohli, K.; Ahmad, S.; Ali, J. Silymarin: A review of pharmacological aspects and bioavailability enhancement approaches. Indian J. Pharmacol. 2007, 39, 172–179. [Google Scholar] [CrossRef]
- Bilia, A.R.; Piazzini, V.; Risaliti, L.; Vanti, G.; Casamonti, M.; Wang, M.; Bergonzi, M.C. Nanocarriers: A Successful Tool to Increase Solubility, Stability and Optimise Bioefficacy of Natural Constituents. Curr. Med. Chem. 2019, in press. [Google Scholar] [CrossRef]
- Wen, Z.; Dumas, T.E.; Schrieber, S.J.; Hawke, R.L.; Fried, M.W.; Smith, P.C. Pharmacokinetics and Metabolic Profile of Free, Conjugated, and Total Silymarin Flavonolignans in Human Plasma after Oral Administration of Milk Thistle Extract. Drug Metab. Dispos. 2007, 36, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Morazzoni, P.; Montalbetti, A.; Malandrino, S.; Pifferi, G. Comparative pharmacokinetics of silipide and silymarin in rats. Eur. J. Drug Metab. Pharmacokinet. 1993, 18, 289–297. [Google Scholar] [CrossRef]
- Ghosh, A.; Biswas, S.; Ghosh, T. Preparation and Evaluation of Silymarin β-cyclodextrin Molecular Inclusion Complexes. J. Young Pharm. 2011, 3, 205–210. [Google Scholar] [CrossRef] [Green Version]
- Li, F.Q.; Hu, J.H. Improvement of the Dissolution Rate of Silymarin by Means of Solid Dispersions. Chem. Pharm. Bull. 2004, 52, 972–973. [Google Scholar] [CrossRef] [Green Version]
- Passerini, N.; Perissutti, B.; Albertini, B.; Franceschinis, E.; Lenaz, D.; Hasa, D.; Locatelli, I.; Voinovich, D. A new approach to enhance oral bioavailability of Silybum marianum dry extract: Association of mechanochemical activation and spray congealing. Phytomedicine 2012, 19, 160–168. [Google Scholar] [CrossRef]
- Younis, N.; Shaheen, M.A.; Abdallah, M.H. Silymarin-loaded Eudragit® RS100 nanoparticles improved the ability of silymarin to resolve hepatic fibrosis in bile duct ligated rats. Biomed. Pharmacother. 2016, 81, 93–103. [Google Scholar] [CrossRef]
- Kumar, N.; Rai, A.; Reddy, N.D.; Raj, P.V.; Jain, P.; Deshpande, P.; Mathew, G.; Kutty, N.G.; Udupa, N.; Rao, C.M. Silymarin liposomes improves oral bioavailability of silybin besides targeting hepatocytes, and immune cells. Pharmacol. Rep. 2014, 66, 788–798. [Google Scholar] [CrossRef]
- He, J.; Hou, S.; Lu, W.; Zhu, L.; Feng, J. Preparation, Pharmacokinetics and Body Distribution of Silymarin-Loaded Solid Lipid Nanoparticles After Oral Administration. J. Biomed. Nanotech. 2007, 3, 195–202. [Google Scholar] [CrossRef]
- Piazzini, V.; Lemmi, B.; D’Ambrosio, M.; Cinci, L.; Luceri, C.; Bilia, A.R.; Bergonzi, M.C. Nanostructured Lipid Carriers as Promising Delivery Systems for Plant Extracts: The Case of Silymarin. Appl. Sci. 2018, 8, 1163. [Google Scholar] [CrossRef]
- Parveen, R.; Baboota, S.; Ali, J.; Ahuja, A.; Vasudev, S.S.; Ahmad, S. Effects of silymarin nanoemulsion against carbon tetrachloride-induced hepatic damage. Arch. Pharm. Res. 2011, 34, 767–774. [Google Scholar] [CrossRef]
- Piazzini, V.; Rosseti, C.; Bigagli, E.; Luceri, C.; Bilia, A.R.; Bergonzi, M.C. Prediction of Permeation and Cellular Transport of Silybum marianum Extract Formulated in a Nanoemulsion by Using PAMPA and Caco-2 Cell Models. Planta Med. 2017, 83, 1184–1193. [Google Scholar] [CrossRef]
- Wu, W.; Wang, Y.; Que, L. Enhanced bioavailability of silymarin by self-microemulsifying drug delivery system. Eur. J. Pharm. Biopharm. 2006, 63, 288–294. [Google Scholar] [CrossRef]
- El-Far, Y.M.; Zakaria, M.M.; Gabr, M.M.; Gayar, A.M.E.; El-Sherbiny, I.M.; Eissa, L.A. A newly developed silymarin nanoformulation as a potential antidiabetic agent in experimental diabetes. Nanomedicine 2016, 11, 2581–2602. [Google Scholar] [CrossRef]
- Soodvilai, S.; Tipparos, W.; Rangsimawong, W.; Patrojanasophon, P.; Soodvilai, S.; Sajomsang, W.; Opanasopit, P. Effects of silymarin-loaded amphiphilic chitosan polymeric micelles on the renal toxicity and anticancer activity of cisplatin. Pharm. Dev. Technol. 2019, in press. [Google Scholar] [CrossRef]
- Kesharwani, S.S.; Kaur, S.; Tummala, H.; Sangamwar, A.T. Multifunctional approaches utilizing polymeric micelles to circumvent multidrug resistant tumors. Colloids Surf. B Biointerfaces 2019, 173, 581–590. [Google Scholar] [CrossRef]
- Lu, Y.; Park, K. Polymeric micelles and alternative nanonized delivery vehicles for poorly soluble drugs. Int. J. Pharm. 2013, 453, 198–214. [Google Scholar] [CrossRef] [Green Version]
- Pepić, I.; Lovrić, J.; Filipović-Grčić, J. How do polymeric micelles cross epithelial barriers? Eur. J. Pharm. Sci. 2013, 50, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Linn, M.; Collnot, E.M.; Djuric, D.; Hempel, K.; Fabian, E.; Kolter, K.; Lehr, C.M. Soluplus® as an effective absorption enhancer of poorly soluble drugs in vitro and in vivo. Eur. J. Pharm. Sci. 2012, 45, 336–343. [Google Scholar] [CrossRef]
- Pignatello, R.; Corsaro, R. Polymeric Nanomicelles of Soluplus® as a Strategy for Enhancing the Solubility, Bioavailability and Efficacy of Poorly Soluble Active Compounds. Curr. Nanomed. 2019, in press. [Google Scholar] [CrossRef]
- Jin, X.; Zhou, B.; Xue, L.; San, W. Soluplus® micelles as a potential drug delivery system for reversal of resistant tumor. Biomed. Pharmacother. 2015, 69, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Yin, T.; Zhang, Y.; Liu, Y.; Chen, Q.; Fu, Y.; Liang, J.; Zhou, J.; Tang, X.; Liu, J.; Huo, M. The efficiency and mechanism of N-octyl-O, N-carboxymethyl chitosan-based micelles to enhance the oral absorption of silybin. Int. J. Pharm. 2018, 536, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lee, S.H.; Feng, S.S. Folate-decorated poly(lactide-co-glycolide)-vitamin E TPGS nanoparticles for targeted drug delivery. Biomaterials 2007, 28, 1889–1899. [Google Scholar] [CrossRef] [PubMed]
- Dintaman, J.M.; Silverman, J.A. Inhibition of P-glycoprotein by D-α-tocopheryl polyethylene glycol 1000 succinate (TPGS). Pharm. Res. 1999, 16, 1550–1556. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Fang, X.; Zhou, D.; Wang, Y.; Chen, M. TPGS-g-PLGA/Pluronic F68 mixed micelles for tanshinone IIA delivery in cancer therapy. Int. J. Pharm. 2014, 476, 185–198. [Google Scholar] [CrossRef]
- Gaucher, G.; Satturwar, P.; Jones, M.C.; Furtos, A.; Leroux, J.C. Polymeric micelles for oral drug delivery. Eur. J. Pharm. Biopharm. 2010, 76, 147–158. [Google Scholar] [CrossRef]
- Francis, M.F.; Cristea, M.; Winnik, F.M. Polymeric micelles for oral drug delivery: Why and how. Pure Appl. Chem. 2004, 76, 1321–1335. [Google Scholar] [CrossRef] [Green Version]
- Win, K.Y.; Feng, S.S. Effects of particle size and surface coating on cellular uptake of polymeric nanoparticles for oral delivery of anticancer drugs. Biomaterials 2005, 26, 2713–2722. [Google Scholar] [CrossRef]
- Dou, J.; Zhang, H.; Liu, X.; Zhang, M.; Zhai, G. Preparation and evaluation in vitro and in vivo of docetaxel loaded mixed micelles for oral administration. Colloids Surf. B Biointerfaces 2014, 114, 20–27. [Google Scholar] [CrossRef]
- Cagel, M.; Bernabeu, E.; Gonzalez, L.; Lagomarsino, E.; Zubillaga, M.; Moretton, M.A.; Chiappetta, D.A. Mixed micelles for encapsulation of doxorubicin with enhanced in vitro cytotoxicity on breast and ovarian cancer cell lines versus Doxil®. Biomed. Pharmacother. 2017, 95, 894–903. [Google Scholar] [CrossRef]
- Bernabeu, E.; Gonzalez, L.; Cagel, M.; Gergic, E.P.; Moretton, M.A.; Chiappetta, D.A. Novel Soluplus®—TPGS mixed micelles for encapsulation of paclitaxel with enhanced in vitro cytotoxicity on breast and ovarian cancer cell lines. Colloids Surf. B Biointerfaces 2016, 140, 403–411. [Google Scholar] [CrossRef]
- Cagel, M.; Tesan, F.C.; Bernabeu, E.; Salgueiro, M.J.; Zubillaga, M.B.; Moretton, M.A.; Chiappetta, D.A. Polymeric mixed micelles as nanomedicines: Achievements and perspectives. Eur. J. Pharm. Biopharm. 2017, 113, 211–228. [Google Scholar] [CrossRef]
- Hu, M.; Zhang, J.; Ding, R.; Fu, Y.; Gong, T.; Zhang, Z. Improved oral bioavailability and therapeutic efficacy of dabigatran etexilate via Soluplus-TPGS binary mixed micelles system. Drug Dev. Ind. Pharm. 2017, 43, 687–697. [Google Scholar] [CrossRef]
- Mehnert, W. Solid lipid nanoparticles Production, characterization and applications. Adv. Drug Deliv. Rev. 2001, 47, 165–196. [Google Scholar] [CrossRef]
- García-Fuentes, M.; Torres, D.; Alonso, M. Design of lipid nanoparticles for the oral delivery of hydrophilic macromolecules. Colloids Surf. B Biointerfaces 2003, 27, 159–168. [Google Scholar] [CrossRef]
- Shen, H.; He, D.; Wang, S.; Ding, P.; Wang, J.; Ju, J. Preparation, characterization, and pharmacokinetics study of a novel genistein-loaded mixed micelles system. Drug Dev. Ind. Pharm. 2018, 44, 1536–1542. [Google Scholar] [CrossRef]
- Kansy, M.; Senner, F.; Gubernator, K. Physicochemical High Throughput Screening: Parallel Artificial Membrane Permeation Assay in the Description of Passive Absorption Processes. J. Med. Chem. 1998, 41, 1007–1010. [Google Scholar] [CrossRef]
- Piazzini, V.; Bigagli, E.; Luceri, C.; Bilia, A.R.; Bergonzi, M.C. Enhanced Solubility and Permeability of Salicis cortex Extract by Formulating as a Microemulsion. Planta Med. 2018, 84, 976–984. [Google Scholar] [CrossRef]
- Piazzini, V.; Cinci, L.; Dambrosio, M.; Luceri, C.; Bilia, A.R.; Bergonzi, M.C. Solid Lipid Nanoparticles and Chitosan-coated Solid Lipid Nanoparticles as Promising Tool for Silybin Delivery: Formulation, Characterization, and In vitro Evaluation. Curr. Drug Deliv. 2018, 16, 142–152. [Google Scholar] [CrossRef]
- Piazzini, V.; Landucci, E.; Graverini, G.; Pellegrini-Giampietro, D.E.; Bilia, A.R.; Bergonzi, M.C. Stealth and Cationic Nanoliposomes as Drug Delivery Systems to Increase Andrographolide BBB Permeability. Pharmaceutics 2018, 10, 128. [Google Scholar] [CrossRef]
- Piazzini, V.; Monteforte, E.; Luceri, C.; Bigagli, E.; Bilia, A.R.; Bergonzi, M.C. Nanoemulsion for improving solubility and permeability of Vitex agnus-castus extract: Formulation and in vitro evaluation using PAMPA and Caco-2 approaches. Drug Deliv. 2017, 24, 380–390. [Google Scholar] [CrossRef]
- Nekkanti, V.; Wang, Z.; Betageri, G.V. Pharmacokinetic Evaluation of Improved Oral Bioavailability of Valsartan: Proliposomes Versus Self-Nanoemulsifying Drug Delivery System. AAPS PharmSciTech 2015, 17, 851–862. [Google Scholar] [CrossRef]
- Huerta, C.; Aberturas, M.D.R.; Molpeceres, J. Nimesulide-loaded nanoparticles for the potential coadjuvant treatment of prostate cancer. Int. J. Pharm. 2015, 493, 152–160. [Google Scholar] [CrossRef]
- Cinci, L.; Luceri, C.; Bigagli, E.; Carboni, I.; Paccosi, S.; Parenti, A.; Guasti, D.; Coronnello, M. Development and characterization of an in vitro model of colorectal adenocarcinoma with MDR phenotype. Cancer Med. 2016, 5, 1279–1291. [Google Scholar] [CrossRef] [Green Version]
- Santos, T.D.; Varela, J.; Lynch, I.; Salvati, A.; Dawson, K.A. Effects of Transport Inhibitors on the Cellular Uptake of Carboxylated Polystyrene Nanoparticles in Different Cell Lines. PLoS ONE 2011, 6, e24438. [Google Scholar] [CrossRef]
- Shah, A.R.; Banerjee, R. Effect of d-α-tocopheryl polyethylene glycol 1000 succinate (TPGS) on surfactant monolayers. Colloids Surf. B Biointerfaces 2011, 85, 116–124. [Google Scholar] [CrossRef]
- Sharma, S.; Sahni, J.K.; Ali, J.; Baboota, S. Effect of high-pressure homogenization on formulation of TPGS loaded nanoemulsion of rutin-pharmacodynamic and antioxidant studies. Drug Deliv. 2014, 22, 541–551. [Google Scholar] [CrossRef]
- Clint, J.H. Micellization of mixed nonionic surface active agents. J. Chem. Soc. Faraday Trans. 1 1975, 71, 1327–1334. [Google Scholar] [CrossRef]
- Rupp, C.; Steckel, H.; Müller, B.W. Solubilization of poorly water-soluble drugs by mixed micelles based on hydrogenated phosphatidylcholine. Int. J. Pharm. 2010, 395, 272–280. [Google Scholar] [CrossRef]
- Piazzini, V.; Landucci, E.; D’Ambrosio, M.; Tiozzo Fasiolo, L.; Cinci, L.; Colombo, G.; Pellegrini-Giampietro, D.E.; Bilia, A.R.; Luceri, C.; Bergonzi, M.C. Chitosan coated human serum albumin nanoparticles: A promising strategy for nose-to-brain drug delivery. Int. J. Biol. Macromol. 2019, 129, 267–280. [Google Scholar] [CrossRef]
- Hou, J.; Sun, E.; Sun, C.; Wang, J.; Yang, L.; Jia, X.B.; Zhang, Z.H. Improved oral bioavailability and anticancer efficacy on breast cancer of paclitaxel via Novel Soluplus®—Solutol® HS15 binary mixed micelles system. Int. J. Pharm. 2016, 512, 186–193. [Google Scholar] [CrossRef]
- Nandni, D.; Vohra, K.K.; Mahajan, R.K. Study of micellar and phase separation behavior of mixed systems of triblock polymers. J. Colloid Interface Sci. 2009, 338, 420–427. [Google Scholar] [CrossRef]
- Tokle, T.; Lesmes, U.; Decker, E.A.; Mcclements, D.J. Impact of dietary fiber coatings on behavior of protein-stabilized lipid droplets under simulated gastrointestinal conditions. Food Funct. 2012, 3, 58–66. [Google Scholar] [CrossRef]
- Liu, J.; Zeng, F.; Allen, C. Influence of serum protein on polycarbonate-based copolymer micelles as a delivery system for a hydrophobic anti-cancer agent. J. Control. Release 2005, 103, 481–497. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, L.; Chu, L.; Han, X.; Zhai, G. Preparation, optimization, characterization and cytotoxicity in vitro of Baicalin-loaded mixed micelles. J. Colloid Interface Sci. 2014, 434, 40–47. [Google Scholar] [CrossRef]
- Bigagli, E.; Cinci, L.; D’Ambrosio, M.; Luceri, C. Pharmacological activities of an eye drop containing Matricaria chamomilla and Euphrasia officinalis extracts in UVB-induced oxidative stress and inflammation of human corneal cells. J. Photochem. Photobiol. B. 2017, 173, 618–625. [Google Scholar] [CrossRef]
- Casamonti, M.; Piazzini, V.; Bilia, A.R.; Bergonzi, M.C. Evaluation of skin permeability of resveratrol loaded liposomes and nanostructured lipid carriers using a Skin Mimic Artificial Membrane (skin-PAMPA). Drug Deliv. Lett. 2019, in press. [Google Scholar] [CrossRef]
Sample Availability: Samples of all compounds are available from the authors. |







| Sample | Average Diameter (nm) | PdI | Zeta Potential (mV) | EE% | LC% |
|---|---|---|---|---|---|
| PNM | 59.7 ± 0.1 | 0.06 ± 0.02 | −5.5 ± 0.6 | - | - |
| MNM | 60.2 ± 2.5 | 0.05 ± 0.01 | −4.7 ± 0.6 | - | - |
| SLM-PNM | 61.3 ± 6.0 | 0.10 ± 0.03 | −4.7 ± 0.5 | 93.0 ± 3.9 | 2.9 ± 0.2 |
| SLM-MNM | 61.5 ± 4.3 | 0.10 ± 0.03 | −4.3 ± 0.5 | 92.9 ± 5.3 | 2.9 ± 0.2 |
| FITC-PNM | 60.6 ± 2.1 | 0.05 ± 0.01 | −6.2 ± 0.3 | 95.3 ± 1.2 | 1.9 ± 0.1 |
| FITC-MNM | 63.3 ± 1.4 | 0.10 ± 0.01 | −5.9 ± 0.2 | 95.7 ± 1.2 | 1.9 ± 0.0 |
| Sample | SLM Solubility (mg/mL) | Sf |
|---|---|---|
| Soluplus | 2.41 ± 0.03 | 6.51 |
| Soluplus/TPGS 20:1 | 2.05 ± 0.07 | 5.54 |
| Soluplus/TPGS 10:1 | 1.89 ± 0.01 | 5.11 |
| Soluplus/TPGS 5:1 | 1.83 ± 0.04 | 4.95 |
| Soluplus/TPGS 4:1 | 1.78 ± 0.04 | 4.81 |
| Soluplus/TPGS 3:1 | 1.64 ± 0.04 | 4.43 |
| Soluplus/TPGS 2:1 | 1.58 ± 0.04 | 4.27 |
| Water | 0.37 ± 0.01 | - |
| Sample | Average Diameter (nm) | PdI | Zeta Potential (mV) | EE% | SLM Precipitate |
|---|---|---|---|---|---|
| SLM-PNM | 57.5 ± 0.5 | 0.07 ± 0.01 | −4.3 ± 0.2 | 93.2 ± 0.1 | NO |
| SLM-MNM | 59.7 ± 0.1 | 0.04 ± 0.01 | −4.5 ± 0.5 | 92.7 ± 0.1 | NO |
| Sample | Average Diameter (nm) | PdI | Zeta Potential (mV) | EE% | SLM Precipitate |
|---|---|---|---|---|---|
| SLM-PNM | 56.7 ± 0.2 | 0.06 ± 0.01 | −4.4 ± 0.2 | 92.7 ± 0.1 | NO |
| SLM-MNM | 57.8 ± 0.2 | 0.06 ± 0.01 | −4.7 ± 0.4 | 92.8 ± 0.1 | NO |
| GF | IF | |||
|---|---|---|---|---|
| Sample | Average Diameter (nm) | PdI | Average Diameter (nm) | PdI |
| SLM-PNM | 58.7 ± 1.1 | 0.12 ± 0.01 | 65.4 ± 2.2 | 0.20 ± 0.02 |
| SLM-MNM | 61.3 ± 0.8 | 0.11 ± 0.01 | 65.3 ± 1.4 | 0.13 ± 0.01 |
| SLM-PNM | SLM-MNM | |||
|---|---|---|---|---|
| Medium | Average Diameter (nm) | PdI | Average Diameter (nm) | PdI |
| PBS 24 h | 68.0 ± 1.1 | 0.08 ± 0.01 | 69.7 ± 3.4 | 0.07 ± 0.01 |
| PBS 48 h | 64.1 ± 1.8 | 0.12 ± 0.02 | 75.8 ± 4.4 | 0.08 ± 0.03 |
| PBS 72 h | 66.6 ± 1.2 | 0.09 ± 0.01 | 72.0 ± 1.4 | 0.11 ± 0.01 |
| PBS + HSA 24 h | 69.6 ± 1.3 | 0.21 ± 0.02 | 75.6 ± 6.3 | 0.26 ± 0.01 |
| PBS + HSA 48 h | 70.6 ± 0.4 | 0.24 ± 0.01 | 74.0 ± 2.1 | 0.24 ± 0.01 |
| PBS + HSA 72 h | 70.9 ± 0.2 | 0.25 ± 0.01 | 70.2 ± 1.1 | 0.25 ± 0.01 |
| Sample | Papp × 10−9 (cm/s) AP-BL | Papp × 10−9 (cm/s) BL-AP | Efflux Ratio |
|---|---|---|---|
| SLM-PNM | 9.10 ± 0.14 | 6.11 ± 1.25 | 0.67 ± 0.15 |
| SLM-MNM | 140.90 ± 15.06 * | 6.16 ± 1.75 | 0.04 ± 0.01 * |
| Free-SLM | N.D. | 17.29 ± 0.30 | N.A. |
| Sample | Soluplus (mg) | TPGS (mg) | SLM (mg) | FITC (mg) |
|---|---|---|---|---|
| PNM | 250 | - | - | - |
| MNM | 238 | 12 | - | - |
| SLM-PNM | 250 | - | 15 | - |
| SLM-MNM | 238 | 12 | 15 | - |
| FITC-PNM | 250 | - | - | 5 |
| FITC-MNM | 238 | 12 | - | 5 |
| Gene | Primer Forward | Primer Reverse | Size |
|---|---|---|---|
| P-gp | CAGAGGCTCTATGACCCCAC | CAACTGGGCCCCTCTCTCTC | 273 |
| GAPDH | CCCTCAAGGGCATCCTGGGCT | GCAGGGACTCCCCAGCAGTGA | 275 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piazzini, V.; D’Ambrosio, M.; Luceri, C.; Cinci, L.; Landucci, E.; Bilia, A.R.; Bergonzi, M.C. Formulation of Nanomicelles to Improve the Solubility and the Oral Absorption of Silymarin. Molecules 2019, 24, 1688. https://doi.org/10.3390/molecules24091688
Piazzini V, D’Ambrosio M, Luceri C, Cinci L, Landucci E, Bilia AR, Bergonzi MC. Formulation of Nanomicelles to Improve the Solubility and the Oral Absorption of Silymarin. Molecules. 2019; 24(9):1688. https://doi.org/10.3390/molecules24091688
Chicago/Turabian StylePiazzini, Vieri, Mario D’Ambrosio, Cristina Luceri, Lorenzo Cinci, Elisa Landucci, Anna Rita Bilia, and Maria Camilla Bergonzi. 2019. "Formulation of Nanomicelles to Improve the Solubility and the Oral Absorption of Silymarin" Molecules 24, no. 9: 1688. https://doi.org/10.3390/molecules24091688
APA StylePiazzini, V., D’Ambrosio, M., Luceri, C., Cinci, L., Landucci, E., Bilia, A. R., & Bergonzi, M. C. (2019). Formulation of Nanomicelles to Improve the Solubility and the Oral Absorption of Silymarin. Molecules, 24(9), 1688. https://doi.org/10.3390/molecules24091688