The Issue of Misidentification of Kojic Acid with Flufuran in Aspergillus flavus
Abstract
:1. Introduction
2. Results
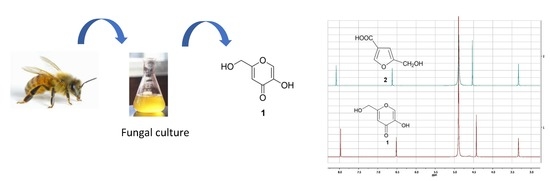
2.1. Comparison of NMR Data Obtained from KA (1) and 5-(Hydroxymethyl)furan-3-carboxylic Acid (2)
2.2. Comparison of Potentiometric, UV, and MS Spectrophotometric Measurements
2.3. Comparison of KA and 5-(Hydroxymethyl)furan-3-carboxylic Acid Derivatives
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Isolation of A. flavus from Bees
4.3. Production and Extraction of KA
4.4. Determination of Protonation Constants
4.5. UV and MS Data of KA and 5-(Hydroxymethyl)furan-3-carboxylic Acid
4.6. Sample Methylation
4.7. Sample Acetylation
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chambergo, F.S.; Valencia, E.Y. Fungal biodiversity to biotechnology. Appl. Microbiol. Biotechnol. 2016, 100, 2567–2577. [Google Scholar] [CrossRef] [PubMed]
- Meyer, V.; Andersen, M.R.; Brakhage, A.A.; Braus, G.H.; Caddick, M.X.; Cairns, T.C.; de Vries, R.P.; Haarmann, T.; Hansen, K.; Hertz-Fowler, C.; et al. Current challenges of research on filamentous fungi in relation to human welfare and a sustainable bio-economy: A white paper. Fungal Biol. Biotechnol. 2016, 3, 6. [Google Scholar]
- Nicoletti, R.; Salvatore, M.M.; Andolfi, A. Secondary metabolites of mangrove-associated strains of Talaromyces. Mar. Drugs 2018, 16, 12. [Google Scholar] [CrossRef] [PubMed]
- Félix, C.; Salvatore, M.M.; DellaGreca, M.; Meneses, R.; Duarte, A.S.; Salvatore, F.; Naviglio, D.; Gallo, M.; Jorrino-Novo, J.V.; Alves, A.; et al. Production of toxic metabolites by two strains of Lasiodiplodia theobromae, isolated from a coconut tree and a human patient. Mycologia 2018, 110, 642–653. [Google Scholar] [CrossRef] [PubMed]
- Marra, R.; Nicoletti, R.; Pagano, E.; DellaGreca, M.; Salvatore, M.M.; Borrelli, F.; Lombardi, N.; Vinale, F.; Woo, S.L.; Andolfi, A. Inhibitory effect of trichodermanone C, a sorbicillinoid produced by Trichoderma citrinoviride associated to the green alga Cladophora sp., on nitrite production in LPS-stimulated macrophages. Nat. Prod. Res. 2018. [Google Scholar] [CrossRef]
- Keller, N.P.; Turner, G.; Bennett, J.W. Fungal secondary metabolism—from biochemistry to genomics. Nat. Rev. Microbiol. 2005, 3, 937. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, M.M.; Nicoletti, R.; Salvatore, F.; Naviglio, D.; Andolfi, A. GC–MS approaches for the screening of metabolites produced by marine-derived Aspergillus. Mar. Chem. 2018, 206, 19–33. [Google Scholar] [CrossRef]
- Samson, R.A.; Visagie, C.M.; Houbraken, J.; Hong, S.B.; Hubka, V.; Klaassen, C.H.W.; Perrone, G.; Seifert, K.A.; Susca, A.; Tanney, J.B.; et al. Taxonomy, identification and nomenclature of the genus Aspergillus. Stud. Mycol. 2014, 78, 141–173. [Google Scholar] [CrossRef]
- Rank, C.; Klejnstrup, M.L.; Petersen, L.M.; Kildgaard, S.; Frisvad, J.C.; Held Gotfredsen, C.; Ostenfeld Larsen, T. Comparative chemistry of Aspergillus oryzae (RIB40) and A. flavus (NRRL 3357). Metabolites 2012, 2, 39–56. [Google Scholar] [CrossRef]
- Cary, J.W.; Gilbert, M.K.; Lebar, M.D.; Majumdar, R.; Calvo, A.M. Aspergillus flavus secondary metabolites: More than just aflatoxins. Food Safety 2018, 6, 7–32. [Google Scholar] [CrossRef]
- Nicoletti, R.; Fiorentino, A. Plant bioactive metabolites and drugs produced by endophytic fungi of Spermatophyta. Agriculture 2015, 5, 918–970. [Google Scholar] [CrossRef]
- Ramírez-Camejo, L.A.; Zuluaga-Montero, A.; Lázaro-Escudero, M.; Hernández-Kendall, V.; Bayman, P. Phylogeography of the cosmopolitan fungus Aspergillus flavus: Is everything everywhere? Fungal Biol. 2012, 116, 452–463. [Google Scholar] [CrossRef] [PubMed]
- Foley, K.; Fazio, G.; Jensen, A.B.; Hughes, W.O. The distribution of Aspergillus spp. opportunistic parasites in hives and their pathogenicity to honey bees. Veter. Microbiol. 2014, 169, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Vojvodic, S.; Jensen, A.B.; James, R.R.; Boomsma, J.J.; Eilenberg, J. Temperature dependent virulence of obligate and facultative fungal pathogens of honeybee brood. Veter. Microbiol. 2011, 149, 200–205. [Google Scholar] [CrossRef]
- Ilyasov, R.; Gaifullina, L.; Saltykova, E.; Poskryakov, A.; Nikolenko, A. Review of the expression of antimicrobial peptide defensin in honey bees Apis mellifera L. J. Apicultural Sci. 2012, 56, 115–124. [Google Scholar] [CrossRef]
- Di Prisco, G.; Annoscia, D.; Margiotta, M.; Ferrara, R.; Varricchio, P.; Zanni, V.; Caprio, E.; Nazzi, F.; Pennacchio, F. A mutualistic symbiosis between a parasitic mite and a pathogenic virus undermines honey bee immunity and health. Proc. Natl. Acad. Sci. 2016, 113, 3203–3208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benoit, J.B.; Yoder, J.A.; Sammataro, D.; Zettler, L.W. Mycoflora and fungal vector capacity of the parasitic mite Varroa destructor (Mesostigmata: Varroidae) in honey bee (Hymenoptera: Apidae) colonies. Int. J. Acarology 2004, 30, 103–106. [Google Scholar] [CrossRef]
- Yang, X.; Cox-Foster, D.L. Impact of an ectoparasite on the immunity and pathology of an invertebrate: Evidence for host immunosuppression and viral amplification. Proc. Natl. Acad. Sci. 2005, 102, 7470–7475. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Jeong, J.H.; Lee, K.T.; Rho, J.R.; Choi, H.D.; Kang, J.S.; Son, B.W. γ-Pyrone derivatives, kojic acid methyl ethers from a marine-derived fungus Altenaria sp. Arch. Pharm. Res. 2003, 26, 532–534. [Google Scholar] [CrossRef]
- Cabrera, G.M.; Roberti, M.J.; Wright, J.E.; Seldes, A.M. Cryptoporic and isocryptoporic acids from the fungal cultures of Polyporus arcularius and P. ciliatus. Phytochemistry 2002, 61, 189–193. [Google Scholar] [CrossRef]
- Alvarez-Ibarra, C.; Quiroga-Feijóo, M.L.; Toledano, E. An analysis of substituent effects on 1H and 13C-NMR parameters of substituted furans. Linear free energy relationships and PM3 semiempirical calculations. J. Chem. Soc., Perkin Trans. 2 1998, 3, 679–690. [Google Scholar] [CrossRef]
- Evidente, A.; Cristinzio, G.; Punzo, B.; Andolfi, A.; Testa, A.; Melck, D. Flufuran, an antifungal 3,5-disubstituted furan produced by Aspergillus flavus Link. Chem. Biodiver. 2009, 6, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Balde, E.S.; Andolfi, A.; Bruyère, C.; Cimmino, A.; Lamoral-Theys, D.; Vurro, M.; Damme, M.V.; Altomare, C.; Mathieu, V.; Kiss, R.; et al. Investigations of fungal secondary metabolites with potential anticancer activity. J. Nat. Prod. 2010, 73, 969–971. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Ma, Y.M.; Li, T.; Wang, J. Structure and activity of secondary metabolites of endophytic fungus S19 strain in Cephalotaxus fortune. Guizhou Nongye Kexue 2014, 42, 152–156. [Google Scholar]
- Ma, Y.M.; Ma, C.C.; Li, T.; Wang, J. A new furan derivative from an endophytic Aspergillus flavus of Cephalotaxus fortunei. Nat. Prod. Res. 2016, 30, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Saldan, N.C.; Almeida, R.T.; Avíncola, A.; Porto, C.; Galuch, M.B.; Magon, T.F.; Pilau, E.J.; Svidzinski, T.I.E.; Oliveira, C.C. Development of an analytical method for identification of Aspergillus flavus based on chemical markers using HPLC-MS. Food Chem. 2018, 241, 113–121. [Google Scholar] [CrossRef]
- Lee, M.; Cho, J.Y.; Lee, Y.G.; Lee, H.J.; Lim, S.I.; Lee, S.Y.; Nam, Y.D.; Moon, J.H. Furan, phenolic, and heptelidic acid derivatives produced by Aspergillus oryzae. Food Sci. Biotechnol. 2016, 25, 1259–1264. [Google Scholar] [CrossRef]
- Yang, X.; Wang, P.; Ma, Y.; Jia, Q. Metabolites of Aspergillus oryzae, an endophytic fungus associated with Lycium ruthenicum Murr. Tianran Chanwu Yanjiu Yu Kaifa 2015, 27, 1554–1557. [Google Scholar]
- Zhang, H.C.; Ma, Y.M.; Liu, R. Antimicrobial materials derived from the endophytic fungus Fusarium sp. of Eucommia ulmoides. Advan. Mater. Res. 2012, 531, 346–349. [Google Scholar] [CrossRef]
- He, S.; Yan, X.; Wang, T. Marine Penicillium dipodomyicola for manufacture of flufuran. 2014, CN 104031845 A 20140910. CN 104031845 A 20140910, 2014. [Google Scholar]
- Elsunni, M.A.; Yang, Z.-D. Secondary metabolites of the endophytic fungi Penicillium polonicum and their monoamine oxidase inhibitory activity. Chem. Nat. Comp. 2018, 54, 1018–1019. [Google Scholar] [CrossRef]
- Zhou, J.; Li, G.; Deng, Q.; Zheng, D.; Yang, X.; Xu, J. Cytotoxic constituents from the mangrove endophytic Pestalotiopsis sp. induce G0/G1 cell cycle arrest and apoptosis in human cancer cells. Nat. Prod. Res. 2018, 32, 2968–2972. [Google Scholar] [CrossRef]
- Nurchi, V.M.; Crisponi, G.; Lachowicz, J.I.; Murgia, S.; Pivetta, T.; Remelli, M.; Rescigno, A.; Niclós-Gutìerrez, J.; González-Pérez, J.M.; Domìnguez-Martìn, A.; et al. Iron (III) and aluminum (III) complexes with hydroxypyrone ligands aimed to design kojic acid derivatives with new perspectives. J. Inorg. Biochem. 2010, 104, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Paterson, R.R.M.; Kemmelmeier, C. Neutral, alkaline and difference ultraviolet spectra of secondary metabolites from Penicillium and other fungi, and comparisons to published maxima from gradient high-performance liquid chromatography with diode-array detection. J. Chromatogr. A 1990, 511, 195–221. [Google Scholar] [CrossRef]
- Klaiklay, S.; Rukachaisirikul, V.; Phongpaichit, S.; Buatong, J.; Preedanon, S.; Sakayaroj, J. Flavodonfuran: A new difuranylmethane derivative from the mangrove endophytic fungus Flavodon flavus PSU-MA201. Nat. Prod. Res. 2013, 27, 1722–1726. [Google Scholar] [CrossRef] [PubMed]
- Kobori, H.; Sekiya, A.; Yasuda, N.; Noguchi, K.; Suzuki, T.; Choi, J.H.; Hirai, H.; Kawagishi, H. Armillariols A to C from the culture broth of Armillaria sp. Tetrahedron Lett. 2013, 54, 5481–5483. [Google Scholar] [CrossRef]
- Ding, L.J.; Gu, B.B.; Jiao, W.H.; Yuan, W.; Li, Y.X.; Tang, W.Z.; Yu, H.B.; Liao, X.J.; Han, B.N.; Li, Z.Y.; et al. New furan and cyclopentenone derivatives from the sponge-associated fungus Hypocrea koningii PF04. Mar. Drugs 2015, 13, 5579–5592. [Google Scholar] [CrossRef]
- Chen, L.L.; Wang, P.; Chen, H.Q.; Guo, Z.K.; Wang, H.; Dai, H.F.; Mei, W.L. New furan derivatives from a mangrove-derived endophytic fungus Coriolopsis sp. J5. Molecules 2017, 22, 261. [Google Scholar] [CrossRef] [PubMed]
- Uchoa, P.K.S.; Pimenta, A.T.; Braz-Filho, R.; de Oliveira, M.D.C.F.; Saraiva, N.N.; Rodrigues, B.S.; Pfenning, L.H.; Abreu, L.M.; Wilke, D.V.; Florêncio, K.G.D.; et al. New cytotoxic furan from the marine sediment-derived fungi Aspergillus niger. Nat. Prod. Res. 2017, 31, 2599–2603. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Pang, X.J.; Xu, L.L.; Zhao, T.; Long, X.Y.; Zhang, Q.Y.; Qin, H.L.; Yang, D.F.; Yang, X.L. Two new alkylated furan derivatives with antifungal and antibacterial activities from the plant endophytic fungus Emericella sp. XL029. Nat. Prod. Res. 2018, 32, 2625–2631. [Google Scholar] [CrossRef] [PubMed]
- Bentley, R. From miso, sake and shoyu to cosmetics: A century of science for kojic acid. Nat. Prod. Rep. 2006, 23, 1046–1062. [Google Scholar] [CrossRef] [PubMed]
- Dembitsky, V.M.; Kilimnik, A. Anti-melanoma agents derived from fungal species. Mathews, J. Pharm. Sci. 2016, 1, 002. [Google Scholar]
- Mohamad, R.; Mohamed, M.S.; Suhaili, N.; Salleh, M.M.; Ariff, A.B. Kojic acid: Applications and development of fermentation process for production. Biotechnol. Mol. Biol. Rev. 2010, 5, 24–37. [Google Scholar]
- Pitt, J.I.; Hocking, A.D.; Glenn, D.R. An improved medium for the detection of Aspergillus flavus and A. parasiticus. J. Appl. Bacteriol. 1983, 54, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Cubero, O.F.; Crespo, A.; Fatehi, J.; Bridge, P.D. DNA extraction and PCR amplification method suitable for fresh, herbarium-stored, lichenized, and other fungi. Plant Syst. Evol. 1999, 216, 243–249. [Google Scholar] [CrossRef]
- Peterson, S.W. Phylogenetic analysis of Aspergillus species using DNA sequences from four loci. Mycologia 2008, 100, 205–226. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, R.; Buommino, E.; De Filippis, A.; Lopez-Gresa, M.P.; Manzo, E.; Carella, A.; Petrazzuolo, M.; Tufano, M.A. Bioprospecting for antagonistic Penicillium strains as a resource of new antitumor compounds. World J. Microbiol. Biotechnol. 2008, 24, 189–195. [Google Scholar] [CrossRef]
- Meloun, M.; Havel, J.; Högfeldt, E. Computation of Solution Equilibria: A Guide to Methods in Potentiometry, Extraction and Spectrophotometry; Ellis Horwood Ltd.: Chichester, UK, 1988. [Google Scholar]
- Gans, P.; Sabatini, A.; Vacca, A. Investigation of equilibria in solution. Determination of equilibrium constants with the HYPERQUAD suite of programs. Talanta 1996, 43, 1739–1753. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 1-9 are available from the authors. |






| Source | Spectroscopic Data | Activity | Ref. |
|---|---|---|---|
| Aspergillus flavus | 1H and 13C-NMR, MS, IR, UV | Antifungal | [22] |
| A. flavus | — | Cytotoxic against human cancer cell lines | [23] |
| A. flavus | Bacteriostatic (Escherichia coli) | [24] 1 | |
| A. flavus2 | 1H and 13C-NMR | Antibacterial, antioxidant | [25] |
| A. flavus | MS | — | [26] |
| Aspergillus oryzae | — | — | [27] |
| A. oryzae | 1 | Antioxidant, antimicrobial | [28] |
| Fusarium sp.3 | 1H and 13C-NMR | Antimicrobial | [29] |
| Penicillium dipodomyicola | 1H and 13C-NMR | — | [30] |
| Penicillium polonicum | — | Monoamine oxidase inhibitor | [31] |
| Pestalotiopsis sp. | 1H and 13C-NMR | Cytotoxic against HepG2 cells | [32] |
| Polyporus ciliatus | P.f., MS, 1H and 13C-NMR, MS, IR, UV | — | [20] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
DellaGreca, M.; De Tommaso, G.; Salvatore, M.M.; Nicoletti, R.; Becchimanzi, A.; Iuliano, M.; Andolfi, A. The Issue of Misidentification of Kojic Acid with Flufuran in Aspergillus flavus. Molecules 2019, 24, 1709. https://doi.org/10.3390/molecules24091709
DellaGreca M, De Tommaso G, Salvatore MM, Nicoletti R, Becchimanzi A, Iuliano M, Andolfi A. The Issue of Misidentification of Kojic Acid with Flufuran in Aspergillus flavus. Molecules. 2019; 24(9):1709. https://doi.org/10.3390/molecules24091709
Chicago/Turabian StyleDellaGreca, Marina, Gaetano De Tommaso, Maria Michela Salvatore, Rosario Nicoletti, Andrea Becchimanzi, Mauro Iuliano, and Anna Andolfi. 2019. "The Issue of Misidentification of Kojic Acid with Flufuran in Aspergillus flavus" Molecules 24, no. 9: 1709. https://doi.org/10.3390/molecules24091709
APA StyleDellaGreca, M., De Tommaso, G., Salvatore, M. M., Nicoletti, R., Becchimanzi, A., Iuliano, M., & Andolfi, A. (2019). The Issue of Misidentification of Kojic Acid with Flufuran in Aspergillus flavus. Molecules, 24(9), 1709. https://doi.org/10.3390/molecules24091709