Evaluation of N-Alkyl-bis-o-aminobenzamide Receptors for the Determination and Separation of Metal Ions by Fluorescence, UV-Visible Spectrometry and Zeta Potential
Abstract
:1. Introduction
2. Results and Discussion
2.1. Fluorescence and UV-Vis Properties of Compounds 1a–5a
2.2. ζ = f (pH) Profiles of the Compounds 1a–5a
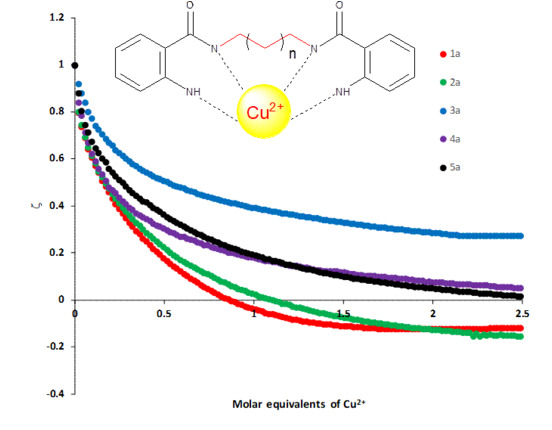
2.3. Study of Metal Ions Recognition
- ζ = Complex zeta potential
- ζH = Free receptor zeta potential
- ζ∞ = Zeta potential in the saturation point induced by complexation
- [G]T = Receptor concentration
- [H]T = Metal ion concentration
- k = Association constant
2.4. Interference Study of the Copper Sensor
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis
4.3. Procedure of Spectrometric Titrations
4.4. Fluorescence Measurements
4.5. ζ = f (pH) Profiles of the Compounds 1a–5a
4.6. Affinity Study of the Compounds 1a–5a with Different Metal Ions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Frederickson, C.J.; Kasarskis, E.J.; Ringo, D.; Frederickson, R.E. A quinoline fluorescence method for visualizing and assaying the histochemically reactive zinc (bouton zinc) in the brain. J. Neurosci. Methods 1987, 20, 91–103. [Google Scholar] [CrossRef]
- Woodroofe, C.C.; Lippard, S.J. A novel two-fluorophore approach to ratiometric sensing of Zn2+. J. Am. Chem. Soc. 2003, 125, 11458–11459. [Google Scholar] [CrossRef]
- Fahrni, C.J.; O’Halloran, T.V. Aqueous Coordination Chemistry of Quinoline-Based Fluorescence Probes for the Biological Chemistry of Zinc. J. Am. Chem. Soc. 1999, 121, 11448–11458. [Google Scholar] [CrossRef]
- Hirano, T.; Kikuchi, K.; Urano, Y.; Higuchi, T.; Nagano, T. Highly Zinc-Selective Fluorescent Sensor Molecules Suitable for Biological Applications. J. Am. Chem. Soc. 2000, 122, 12399–12400. [Google Scholar] [CrossRef]
- Kiyose, K.; Kojima, H.; Urano, Y.; Nagano, T. Development of a Ratiometric Fluorescent Zinc Ion Probe in Near-Infrared Region, Based on Tricarbocyanine Chromophore. J. Am. Chem. Soc. 2006, 128, 6548–6549. [Google Scholar] [CrossRef] [PubMed]
- Antina, E.V.; Bumagina, N.A.; V’yugin, A.I.; Solomonov, A.V. Fluorescent indicators of metal ions based on dipyrromethene platform. Dyes Pigm. 2017, 136, 368–381. [Google Scholar] [CrossRef]
- Mei, Y.; Bentley, P.A.; Wang, W. A selective and sensitive chemosensor for Cu2+ based on 8-hidroxyquinoline. Tetrahedron Lett. 2006, 47, 2477–2479. [Google Scholar] [CrossRef]
- Atilgan, S.; Kutuk, I.; Ozdemir, T. A near IR di-styryl BODIPY-Based radiometric fluorescence chemosensor for Hg(II). Tetrahedron Lett. 2010, 51, 892–894. [Google Scholar] [CrossRef]
- Pina-Luis, G.; Ochoa-Teran, A.; Rivero, I. Solid phase synthesis of N-alkyl-bis-o-aminobenzamides for metal ion sensing based on a fluorescent dansyl plataform. J. Comb. Chem. 2003, 11, 83–90. [Google Scholar] [CrossRef]
- Wang, J.-M.; Jiang, X.; Zhang, Y.; Zhu, Y.-M.; Shen, J.-K. Palladium-catalyzed synthesis of 4H-benzo[d][1,3]oxazin-4-ones and N-(2-cyanophenyl)benzamides via tert-butyl isocyanide insertion. Tetrahedron Lett. 2015, 56, 2349–2354. [Google Scholar] [CrossRef]
- Wang, X.-M.; Xin, M.-H.; Xu, J.; Kang, B.-R.; Lin, Y.; Lu, S.-M.; Zhang, S.Q. Synthesis and antitumor activities evaluation of m-(4-morpholinoquinazolin-2-yl)benzamides in vitro and in vivo. Eur. J. Med. Chem. 2015, 96, 382–395. [Google Scholar] [CrossRef] [PubMed]
- Khanfar, M.A.; Quinti, L.; Wang, H.; Choi, S.H.; Kazantsev, A.G.; Silverman, R.B. Development and characterization of 3-(benzylsulfonamido)benzamides as potent and selective SIRT2 inhibitors. Eur. J. Med. Chem. 2014, 76, 414–426. [Google Scholar] [CrossRef]
- John, C.S.; Vilner, B.J.; Geyer, B.C.; Moody, T.; Bowen, W.D. Targeting sigma receptor-binding benzamides as in Vivo diagnostic and therapeutic agents for human prostate tumors. Cancer Res. 1999, 59, 4578–4583. [Google Scholar] [PubMed]
- Mahdavi, M.; Lavi, M.M.; Yekta, R.; Moosavi, M.A.; Nobarani, M.; Balalaei, S.; Arami, S.; Rashidi, M.R. Evaluation of the cytotoxic, apoptosis inducing activity and molecular docking of spiroquinazolinone benzamide derivatives in MCF-7 breast cancer cells. Chem. Biol. Interact. 2016, 25, 232–242. [Google Scholar] [CrossRef]
- Jia, J.; Gu, Z.; Li, R.; Huang, M.; Xu, C.; Wang, Y.; Xing, G.; Huang, Y. Design and synthesis of fluorescent sensors for zinc ion derived from 2-aminobenzamide. J. Org. Chem. 2011, 24, 4609–4615. [Google Scholar] [CrossRef]
- Chandross, E.A.; Dempster, C.J. Intramolecular excimer formation and fluorescence quenching in dinaphthylalkanes. J. Am. Chem. Soc. 1970, 92, 3586–3593. [Google Scholar] [CrossRef]
- Schazmann, B.; Alhashimy, N.; Diamond, D. Chloride Selective Calix [4]arene Optical Sensor Combining Urea Functionality with Pyrene Excimer Transduction. J. Am. Chem. Soc. 2006, 128, 8607–8614. [Google Scholar] [CrossRef]
- Sarkar, S.; Roy, S.; Sikdar, A.; Saha, R.N.; Panj, S.S. A pyrene-based simple but highly selective fluorescence sensor for Cu2+ ions via a static excimer mechanism. Analyst 2013, 138, 7119–7126. [Google Scholar] [CrossRef]
- Moromichi, I. Excel Worksheets for Spectrometry; Universidad de Sonora: Hermosillo, Mexico, 2009. [Google Scholar]
- Awual, M.R.; Hasan, M.M. Colorimetric detection and removal of copper(II) ions from wastewater samples using tailor-made composite adsorbent. Sens. Actuators B 2015, 206, 692–700. [Google Scholar] [CrossRef]
- Awual, M.R.; Hasan, M.M.; Shahat, A. Functionalized novel mesoporous adsorbent for selective lead(II) ionsmonitoring and removal from wastewater. Sens. Actuators B 2014, 203, 854–863. [Google Scholar] [CrossRef]
- Price, C.; Carroll, J.; Clare, T.L. Chemoresistive and photonic hydrogel sensors of transition metal ions via Hofmeister series principles. Sens. Actuators B 2018, 256, 870–877. [Google Scholar] [CrossRef]
- Yoo, J.; Ryu, U.; Kwon, W.; Min Choi, K. A multi-dye containing MOF for the ratiometric detection and simultaneous removal of Cr2O72− in the presence of interfering ions. Sens. Actuators B. Chem. 2019, 283, 426–433. [Google Scholar] [CrossRef]
- Ramachamdram, B.; Saroja, G.; Sankaran, N.B.; Samanta, A. Unusually High Fluorescence Enhancement of Some 1,8-Naphthalimide Derivatives Induced by Transition Metal Salts. J. Phys. Chem. B 2000, 104, 11824. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available. |










| Compound | IEP | pka Fluorescence Values |
|---|---|---|
| 1a | 6 | 1.83 |
| 2a | 6 | 2.69 |
| 3a | 6 | 2.60 |
| 4a | 7 | 3.32 |
| 5a | 7 | 2.68 |
| Derivative/Parameter (nm) | λA | λF | λA − λF | ΦF |
|---|---|---|---|---|
| 1a | 314 | 416 | 102 | 0.0026 |
| 2a | 314 | 416 | 102 | 0.0023 |
| 3a | 312 | 415 | 103 | 0.0315 |
| 4a | 314 | 416 | 102 | 0.0015 |
| 5a | 316 | 418 | 102 | 0.0049 |
| Compound | kf |
|---|---|
| 1a | 3.32 × 105 |
| 2a | 3.28 × 105 |
| 3a | 2.28 × 107 |
| 4a | -- |
| 5a | 1.10 × 109 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez-Quiroz, M.; Aguilar-Martinez, X.E.; Oropeza-Guzman, M.T.; Valdez, R.; Lopez-Maldonado, E.A. Evaluation of N-Alkyl-bis-o-aminobenzamide Receptors for the Determination and Separation of Metal Ions by Fluorescence, UV-Visible Spectrometry and Zeta Potential. Molecules 2019, 24, 1737. https://doi.org/10.3390/molecules24091737
Martinez-Quiroz M, Aguilar-Martinez XE, Oropeza-Guzman MT, Valdez R, Lopez-Maldonado EA. Evaluation of N-Alkyl-bis-o-aminobenzamide Receptors for the Determination and Separation of Metal Ions by Fluorescence, UV-Visible Spectrometry and Zeta Potential. Molecules. 2019; 24(9):1737. https://doi.org/10.3390/molecules24091737
Chicago/Turabian StyleMartinez-Quiroz, Marisela, Xiomara E. Aguilar-Martinez, Mercedes T. Oropeza-Guzman, Ricardo Valdez, and Eduardo A. Lopez-Maldonado. 2019. "Evaluation of N-Alkyl-bis-o-aminobenzamide Receptors for the Determination and Separation of Metal Ions by Fluorescence, UV-Visible Spectrometry and Zeta Potential" Molecules 24, no. 9: 1737. https://doi.org/10.3390/molecules24091737
APA StyleMartinez-Quiroz, M., Aguilar-Martinez, X. E., Oropeza-Guzman, M. T., Valdez, R., & Lopez-Maldonado, E. A. (2019). Evaluation of N-Alkyl-bis-o-aminobenzamide Receptors for the Determination and Separation of Metal Ions by Fluorescence, UV-Visible Spectrometry and Zeta Potential. Molecules, 24(9), 1737. https://doi.org/10.3390/molecules24091737