Carbazole- and Triphenylamine-Substituted Pyrimidines: Synthesis and Photophysical Properties
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis
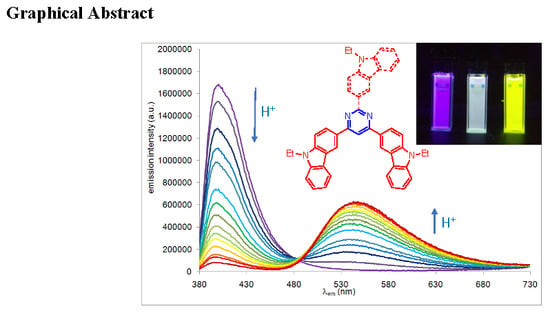
2.2. Photophysical Properties in Solution
2.3. Photophysical Properties in Solid State
3. Materials and Methods
3.1. General Information
3.2. General Procedure for Suzuki Cross-coupling Reactions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Achelle, S.; Rodríguez-López, J.; Robin-le Guen, F. Photoluminescence properties of aryl- arylvinyl- and arylethynylpyrimidine derivatives. ChemistrySelect 2018, 3, 1852–1885. [Google Scholar] [CrossRef]
- Aranda, A.I.; Achelle, S.; Hammerer, F.; Mahuteau-Betzer, F.; Teulade-Fichou, M.-P. Vinyl-diazine triphenylamines and their N-methylated derivatives: synthesis, photophysical properties and application for staining DNA. Dyes Pigm. 2012, 95, 400–407. [Google Scholar] [CrossRef]
- Lipunova, G.N.; Nosova, E.V.; Charushin, V.N.; Chupakhin, O.N. Functionalized quinazolines and pyrimidines for optoelectronics. Curr. Org. Synth. 2018, 15, 793–814. [Google Scholar] [CrossRef]
- Achelle, S.; Plé, N. Pyrimidine ring as building block for the synthesis of functionalized π-conjugated materials. Curr. Org. Synth. 2012, 9, 163–187. [Google Scholar] [CrossRef]
- Li, L.; Ge, J.; Wu, H.; Xu, Q.-H.; Yao, S.Q. Organelle-specific detection of phosphatase activities with two-photon fluorogenic probes in cells and tissues. J. Am. Chem. Soc. 2012, 134, 12157–12167. [Google Scholar] [CrossRef] [PubMed]
- Na, Z.; Li, L.; Uttamchandani, M.; Yao, S.Q. Microarray-guided discovery of two-photon (2P) small molecule probes for live-cell imaging of cysteinyl cathepsin activities. Chem. Commun. 2012, 48, 7304–7306. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Tian, X.; Hu, Z.; Brommesson, C.; Wu, J.; Zhou, H.; Yang, J.; Sun, Z.; Tian, Y.; Uvdal, K. Nonlinear optical response and two-photon biological applications of a new family of imidazole-pyrimidine derivatives. Dyes Pigm. 2016, 126, 286–295. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, H.-L.; Liu, J.; Zhao, Y.-D.; Luo, Q.-M.; Huang, Z.-L. Novel pyrimidine-based amphiphilic molecules: synthesis, spectroscopic properties and applications in two-photon fluorescence imaging. J. Mater. Chem. 2007, 17, 2921–2929. [Google Scholar] [CrossRef]
- Malval, J.-P.; Achelle, S.; Bodiou, L.; Spangenberg, A.; Gomez, L.C.; Soppera, O.; Robin-le Guen, F. Two-photon absorption in a conformationally twisted D-π-A oligomer: a synergic photosensitizing approach for multiphoton lithography. J. Mater. Chem. C 2014, 4, 7869–7880. [Google Scholar] [CrossRef]
- Li, L.; Tian, Y.; Yang, J.-X.; Sun, P.-P.; Wu, J.-Y.; Zhou, H.-P.; Zhang, S.-Y.; Jin, B.-K.; Xing, X.-J.; Wang, C.-K.; et al. Facile synthesis and systematic investigations of a series of novel bent-shaped two-photon absorption chromophores based on pyrimidine. Chem. Asian J. 2009, 4, 668–680. [Google Scholar] [CrossRef]
- Serevičius, T.; Bučiūnas, T.; Bucevičius, J.; Dodonova, J.; Tumkevičius, S.; Kazlauskas, K.; Juršėnas, S. Room-temperature phosphorescence vs. thermally activated delayed fluorescence in cabazole pyrimidine cored compounds. J. Mater. Chem. C 2018, 6, 11128–11136. [Google Scholar] [CrossRef]
- Li, B.; Li, Z.; Hu, T.; Zhang, Y.; Wang, Y.; Yi, Y.; Guo, F.; Zhao, L. Highly efficient blue organic light-emitting diodes from pyrimidine-based thermally activated delayed fluorescence emitters. J. Mater. Chem. C 2018, 6, 2351–2359. [Google Scholar] [CrossRef]
- Komatsu, R.; Sasabe, H.; Kido, J. Recent progress of pyrimidine derivatives for high-performance organic light-emitting devices. J. Photonics Energy 2018, 8, 032108. [Google Scholar] [CrossRef]
- Komatsu, R.; Ohsawa, T.; Sasabe, H.; Nakao, K.; Hayasaka, Y.; Kido, J. Manipulating the electronic excited state energies of pyrimidine-based thermally activated delayed fluorescence emitters to realize efficient deep-blue emission. ACS Appl. Mater. Interfaces 2017, 9, 4742–4749. [Google Scholar] [CrossRef] [PubMed]
- Nakao, K.; Sasabe, H.; Komatsu, R.; Hayasaka, Y.; Ohsawa, T.; Kido, J. Unlocking the potential of pyrimidine conjugate emitters to realize high-performance organic light-emitting devices. Adv. Opt. Mater. 2017, 5, 1600843. [Google Scholar] [CrossRef]
- Durand, R.J.; Gauthier, S.; Achelle, S.; Groizard, T.; Kahlal, S.; Saillard, J.-Y.; Barsella, A.; le Poul, P.; Robin-le Guen, F. Push-pull D-π-Ru-π-A chromophores: synthesis and electrochemical, photophysical and second-order nonlinear optical properties. Dalton Trans. 2018, 47, 3965–3975. [Google Scholar] [CrossRef] [PubMed]
- Achelle, S.; Kahlal, S.; Barsella, A.; Saillard, J.-Y.; Che, X.; Vallet, J.; Bureš, F.; Caro, B.; Robin-le Guen, F. Improvement of the quadratic non-linear optical properties of pyrimidine chromophores by N-methylation and tungsten pentacarbonyl complexation. Dyes Pigm. 2015, 113, 562–570. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Liu, D.; Wang, T.; Li, P.; Bridgmohan, C.N.; Li, W.; Lu, T.; Hu, W.; Wang, L.; Zhou, X. Charge-separated sensitizers with enhanced intramolecular charge transfer for dye-sensitized solar cells: Insight from structure-performance relationships. Org. Electron. 2018, 61, 35–45. [Google Scholar] [CrossRef]
- Verbitskiy, E.V.; Cheprakova, E.M.; Subbotina, J.O.; Schepochkin, A.V.; Slepukhin, P.A.; Rusinov, G.L.; Charushin, V.N.; Chupakhin, O.N.; Makarova, N.I.; Metelitsa, A.V.; et al. Synthesis, spectral and electrochemical properties of pyrimidine-containing dyes as photosensitizers for dye-sensitized solar cells. Dyes Pigm. 2014, 100, 201–204. [Google Scholar] [CrossRef]
- Achelle, S.; Robin-le Guen, F. Emission properties of diazine chromophores: structure-properties relationship. J. Photochem. Photobiol. A 2017, 348, 281–286. [Google Scholar] [CrossRef]
- Schomaker, J.M.; Delia, T.J. Arylation of halogenated pyrimidines via a Suzuki coupling reaction. J. Org. Chem. 2001, 66, 7125–7128. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.C.; Handy, S.T. One-pot double Suzuki couplings of dichloropyrimidines. Synthesis 2010, 2721–2724. [Google Scholar]
- Delia, T.J.; Schomaker, J.M.; Kalinda, A.S. The synthesis of substituted phenylpyrimidines via Suzuki coupling reactions. J. Heterocycl. Chem. 2006, 43, 127–131. [Google Scholar] [CrossRef]
- Achelle, S.; Ramondenc, Y.; Marsais, F.; Plé, N. Star- and banana-shaped oligomers with a pyrimidine core: synthesis and light-emitting properties. Eur. J. Org. Chem. 2008, 3129–3140. [Google Scholar] [CrossRef]
- Littke, A.F.; Fu, G.C. Palladium-catalyzed coupling reactions of aryl chlorides. Angew. Chem. Int. Ed. 2002, 41, 4176–7211. [Google Scholar] [CrossRef]
- Lartia, R.; Allain, C.; Bordeau, G.; Schmidt, F.; Fiorini-Debuisschert, C.; Charra, F.; Teulade-Fichou, M.-P. Synthetic strategies to derivatizable triphenylamine displaying high two-photon absorption. J. Org. Chem. 2008, 73, 1732–1744. [Google Scholar] [CrossRef]
- Katan, C.; Charlot, M.; Mongin, O.; Le Droumaguet, C.; Jouikov, V.; Terenziani, F.; Badaeva, E.; Tretiak, S.; Blanchard-Desce, M. Simultaneous control of emission localization and two-photon absorption efficiency in dissymmetrical chromophores. J. Phys. Chem. B 2010, 114, 3152–3169. [Google Scholar] [CrossRef]
- Merkt, F.K.; Höwedes, S.P.; Gers-Panther, C.F.; Gruber, I.; Janiak, C.; Müller, T.J.J. Three-component activation/alkynylation/cyclocondensation (AACC) synthesis of enhanced emission solvatochromic 3-ethynylquinoxalines. Chem. Eur. J. 2018, 24, 8114–8125. [Google Scholar] [CrossRef]
- Panthi, K.; Adhikari, R.M.; Kinstle, T.H. Aromatic fumaronitrile core-based donor-linker-acceptor-linker-donor (D-pi-A-pi-D) compounds: synthesis and photophysical properties. J. Phys. Chem. A 2010, 114, 4542–4549. [Google Scholar] [CrossRef]
- Itami, K.; Yamazaki, D.; Yoshida, J.-I. Pyrimidine-core extended π-systems: general synthesis and interesting fluorescent properties. J. Am. Chem. Soc. 2004, 126, 15396–15397. [Google Scholar] [CrossRef]
- Muraoka, H.; Obara, T.; Ogawa, S. Systematic synthesis, comparative studies of the optical properties, and the ICT-based sensor properties of a series of 2,4,6-tri(5-aryl-2-thienyl)pyrimidines with the D-π-A system. Tetrahedron Lett. 2016, 57, 3011–3015. [Google Scholar] [CrossRef]
- Fecková, M.; le Poul, P.; Robin-le Guen, F.; Roisnel, T.; Pytela, O.; Klikar, M.; Bureš, F.; Achelle, S. 2,4-Distyryl- and 2,4,6-tristyrylpyrimidines: synthesis and photophysical properties. J. Org. Chem. 2018, 83, 11712–11726. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Aguilar, J.; Vidal, M.; Pastenes, C.; Aliaga, C.; Caroli Rezende, M.; Domínguez, M. The solvatofluorochromism of 2,4,6-triarylpyrimidine derivatives. Photochem. Photobiol. 2018, 94, 1100–1108. [Google Scholar] [CrossRef] [PubMed]
- Achelle, S.; Rodríguez-López, J.; Katan, C.; Robin-le Guen, F. Luminescence behavior of protonated methoxy-substituted diazine derivatives: toward white light emission. J. Phys. Chem. C 2016, 120, 26986–26995. [Google Scholar] [CrossRef]
- Achelle, S.; Rodríguez-López, J.; Cabon, N.; Robin-le Guen, F. Protonable pyrimidine derivative for white light emission. RSC Adv. 2015, 5, 107396–107399. [Google Scholar] [CrossRef]
- Telore, R.D.; Satam, M.A.; Sekar, N. Push-pull fluorophores with viscosity dependent and aggregation induced emissions insensitive to polarity. Dyes Pigm. 2015, 122, 359–367. [Google Scholar] [CrossRef]
- Cvejn, D.; Michael, E.; Polyzos, I.; Almonasy, N.; Pytela, O.; Klikar, M.; Mikysek, T.; Giannetas, V.; Fakis, M.; Bureš, F. Modulation of (non)linear optical properties in tripodal molecules by variation of the peripheral cyano acceptor moieties and the π-spacer. J. Mater. Chem. C 2015, 3, 7345–7355. [Google Scholar] [CrossRef] [Green Version]
- Le Droumaguet, C.; Sourdon, A.; Genin, E.; Mongin, O. Blanchard-Desce Two-photon polarity probes from octupolar fluorophores: synthesis, structure-properties relationships, and use in cellular imaging. Chem. Asian J. 2013, 8, 2984–3001. [Google Scholar] [CrossRef]
- Dumat, B.; Bordeau, G.; Faurel-Paul, E.; Mahuteau-Betzer, F.; Saetel, N.; Metge, G.; Fiorini-Debuisschert, C.; Charra, F.; Teulade-Fichou, M.-P. DNA switches on the two-photon efficiency of an ultrabright triphenylamine fluorescent probe specific of AT regions. J. Am. Chem. Soc. 2013, 135, 12697–12706. [Google Scholar] [CrossRef]
- Kwon, O.; Barlow, S.; Odom, S.A.; Beverina, L.; Thompson, N.J.; Zojer, E.; Brédas, J.-L.; Marder, S.R. Aromatic amines: a comparison of electron-donor strengths. J. Phys. Chem. A 2005, 109, 9346–9352. [Google Scholar] [CrossRef]
- Tydlitát, J.; Achelle, S.; Rodríguez-López, J.; Pytela, O.; Mikýsek, T.; Cabon, N.; Robin-le Guen, F.; Miklík, D.; Růžičková, Z.; Bureš, F. Photophysical properties of acid-responsive triphenylamine derivatives bearing pyridine fragments: towards white light emission. Dyes Pigm. 2017, 146, 467–478. [Google Scholar] [CrossRef]
- Parthasarathy, V.; Fery-Forgues, S.; Campioli, E.; Recher, G.; Terenziani, F.; Blanchard-Desce, M. Dipolar versus octupolar triphenylamine-based fluorescent organic nanoparticles as brillant one- and two-photon emitters for (bio)imaging. Small 2011, 7, 3219–3229. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Huang, J.; Kong, L.; Tian, Y.; Yang, J. Branched triphenylamine luminophores: aggregation-induced fluorescence emission, and tunable near-infrared solid-state fluorescence characteristics via external mechanical stimuli. Dyes Pigm. 2018, 151, 140–149. [Google Scholar] [CrossRef]
- Weng, J.; Mei, Q.; Fan, Q.; Ling, Q.; Tong, B.; Huang, W. Bipolar luminescent materials containing pyrimidine terminals: synthesis, photophysical properties and a theoretical study. RSC Adv. 2013, 3, 21877–21887. [Google Scholar] [CrossRef]
- Kato, S.-I.; Yamada, Y.; Hiyoshi, H.; Umezu, K.; Nakamura, Y. Series of carbazole-pyrimidine conjugates: syntheses and electronic, photophysical, and electrochemical properties. J. Org. Chem. 2015, 80, 9076–9090. [Google Scholar] [CrossRef] [PubMed]
- Reichardt, C. Solvatochromic dyes as solvent polarity indicators. Chem. Rev. 1994, 94, 2319–2358. [Google Scholar] [CrossRef]
- Cerón-Carrasco, J.P.; Jacquemin, D.; Laurence, C.; Planchat, A.; Reichardt, C.; Sraïdi, K. Solvent polarity scales: determination of new Et(30) values for 84 organic solvents. J. Phys. Org. Chem. 2014, 27, 512–518. [Google Scholar] [CrossRef]
- Achelle, S.; Nouira, I.; Pfaffinger, B.; Ramondenc, Y.; Plé, N.; Rodríguez-López, J. V-shaped 4,6-bis(arylvinyl)pyrimidine oligomers: synthesis and optical properties. J. Org. Chem. 2009, 74, 3711–3717. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, Z.; Zhang, H.; Wang, Y. A novel approach towards white photoluminescence and electroluminescence by controlled protonation of a blue fluorophore. Chem. Commun. 2013, 49, 10001–10003. [Google Scholar] [CrossRef]
- Liu, C.; Wu, Y.; Qiu, J. Efficient synthesis of 4-heteroaryl-substituted triphenylamine derivatives via a ligand-free Suzuki reaction. Appl. Organomet. Chem. 2011, 25, 862–866. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 1 and 2 are available from the authors. |







| Compd | λabs (nm) | ε (mM−1 cm−1) | λem (nm) | ΦF | Stokes Shift (cm−1) |
|---|---|---|---|---|---|
| 1a | 356, 301 | 21.7, 13.3 | 456 | 0.83 | 6160 |
| 1b | 388, 301 | 52.0, 26.4 | 486 | 0.86 | 5200 |
| 1c | 376, 299, 293 | 76.6, 36.7, 36.9 | 475 | 0.52 | 5540 |
| 2a | 323, 284, 276 (sh) | 22.6, 51.6, 33.8 | 382 | 0.32 | 4780 |
| 2b | 360, 348, 284 | 43.2, 48.2, 50.1 | 407 | 0.77 | 3210 |
| 2c | 367 (sh) 339, 299, 282 (sh) | 20.4, 69.2, 84.2, 64.1 | 398 | 0.35 | 2120 |
| Compd | n-Heptane 30.9 a | Toluene 33.9 a | 1,4-Dioxane 36.0 a | THF 37.4 a | CHCl3 39.1 a | CH2Cl2 40.7 a | Acetone 42.2 a | MeCN 45.6 a |
|---|---|---|---|---|---|---|---|---|
| 1a | 406 | 420 | 429 | 449 | 450 | 456 | 462 | 477 |
| 1b | 418 | 437 | 444 | 464 | 478 | 486 | 498 | 518 |
| 1c | 413 | 432 | 438 | 466 | 466 | 475 | 492 | 508 |
| 2a | 354/373 | 361/378 | 362/379 | 379 | 383 | 382 | 382 | 389 |
| 2b | 371 | 383 | 384 | 395 | 406 | 407 | 413 | 424 |
| 2c | 379 | 384 | 386 | 385 | 398 | 398 | 407 | 415 |
| Compd | λabs (nm) | ε (mM−1 cm−1) | λem (nm) | ΦF | Stokes Shift (cm−1) |
|---|---|---|---|---|---|
| 1a | 424, 373 (sh), 268 | 17.2, 9.3, 30.5 | - | - | - |
| 1b | 488 | 48.3 | 651 | <0.01 | 5130 |
| 1c | 486, 375, 274 | 68.2, 30.4, 88.5 | - | - | - |
| 2a | 396 | 27.3 | - | - | - |
| 2b | 444, 390, 285 | 63.9, 28.0, 64.3 | 519 | 0.63 | 3250 |
| 2c | 443, 330 | 41.9, 29.0 | 552 | 0.45 | 4460 |
| Compd | Chromaticity Coordinates (x, y) | ||
|---|---|---|---|
| Neutral Form | Protonated Form | Mixture of Neutral and Protonated Forms | |
| 1aa | (0.15, 0.15) | - | - |
| 1bb | (0.18, 0.33) | (0.62, 0.37) | (0.32, 0.34) c |
| 1ca | (0.16, 0.25) | - | - |
| 2aa | (0.18, 0.04) | - | - |
| 2ba | (0.16, 0.03) | (0.29, 0.59) | (0.24, 0.39) d |
| 2ca | (0.17, 0.04) | (0.42, 0.56) | (0.30, 0.34) e |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Achelle, S.; Rodríguez-López, J.; Larbani, M.; Plaza-Pedroche, R.; Robin-le Guen, F. Carbazole- and Triphenylamine-Substituted Pyrimidines: Synthesis and Photophysical Properties. Molecules 2019, 24, 1742. https://doi.org/10.3390/molecules24091742
Achelle S, Rodríguez-López J, Larbani M, Plaza-Pedroche R, Robin-le Guen F. Carbazole- and Triphenylamine-Substituted Pyrimidines: Synthesis and Photophysical Properties. Molecules. 2019; 24(9):1742. https://doi.org/10.3390/molecules24091742
Chicago/Turabian StyleAchelle, Sylvain, Julián Rodríguez-López, Massinissa Larbani, Rodrigo Plaza-Pedroche, and Françoise Robin-le Guen. 2019. "Carbazole- and Triphenylamine-Substituted Pyrimidines: Synthesis and Photophysical Properties" Molecules 24, no. 9: 1742. https://doi.org/10.3390/molecules24091742
APA StyleAchelle, S., Rodríguez-López, J., Larbani, M., Plaza-Pedroche, R., & Robin-le Guen, F. (2019). Carbazole- and Triphenylamine-Substituted Pyrimidines: Synthesis and Photophysical Properties. Molecules, 24(9), 1742. https://doi.org/10.3390/molecules24091742