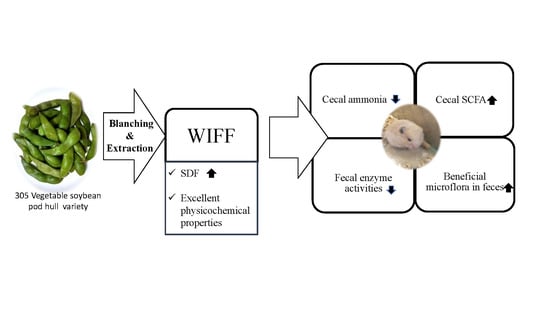
Physicochemical Properties and Intestinal Health Promoting Water-Insoluble Fiber Enriched Fraction Prepared from Blanched Vegetable Soybean Pod Hulls
Abstract
:1. Introduction
2. Results and Discussion
2.1. Proximate Analysis
2.2. Monosaccharide Composition
2.3. Physicochemical Properties
2.4. Animal Growth
2.5. Cecal pH Value, Cecal Ammonia, and Fecal Ammonia
2.6. Activities of Bacterial Enzymes in Feces
2.7. SCFA Production
2.8. Fecal Microbiota Composition
2.9. Strengths and Limitations
3. Materials and Methods
3.1. Sample Preparation and Blanching Process
3.2. Proximate Composition
3.3. Preparation of Water-Insoluble Fiber Enriched Fraction (WIFF)
3.4. Measurement of Dietary Fiber Content
3.5. Determination of Monosaccharide Composition
3.6. Animal and Diet
3.7. Determination of Cecal pH and Ammonia
3.8. Determination of Bacterial Enzyme Activities in Feces
3.9. Determination of Short-Chain Fatty Acid (SCFA) Concentration
3.10. Determination of Fecal Microbiota
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chater, P.I.; Wilcox, M.D.; Pearson, P.J.; Brownlee, I.A. The impact of dietary fibres on the physiological processes governing small intestinal digestive processes. Bioact. Carbohydr. Dietary Fibre 2015, 6, 117–132. [Google Scholar] [CrossRef]
- Zhao, J.; Bai, Y.; Tao, S.; Zhang, G.; Wang, J.; Liu, L.; Zhang, S. Fiber-rich foods affected gut bacterial community and short-chain fatty acids production in pig model. J. Funct. Foods 2019, 57, 266–274. [Google Scholar] [CrossRef]
- Luo, X.L.; Wang, Q.; Zheng, B.D.; Lin, L.M.; Chen, B.Y.; Zheng, Y.F.; Xiao, J.B. Hydration properties and binding capacities of dietary fibers from bamboo shoot shell and its hypolipidemic effects in mice. Food Chem Toxicol. 2017, 109, 1003–1009. [Google Scholar] [CrossRef]
- Wang, X.L.; Wang, X.; Jiang, H.; Cai, C.; Li, G.Y.; Hao, J.J.; Yu, G.L. Marine polysaccharides attenuate metabolic syndrome by fermentation products and altering gut microbiota: An overview. Carbohydr. Polym. 2018, 195, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.L.; Wang, Q.; Fang, D.Y.; Zhuang, W.J.; Chen, C.H.; Jiang, W.T.; Zheng, Y.F. Modification of insoluble dietary fibers from bamboo shoot shell: Structural characterization and functional properties. Int. J. Biol. Macromol. 2018, 120, 1461–1467. [Google Scholar] [CrossRef] [PubMed]
- Martin-Sanchez, A.M.; Sarra, C.; Vilella-Espla, J.; Ben-Abda, J.; Kuri, V.; Perez-Alvarez, J.A.; Sayas-Barbera, E. Characterization of novel intermediate food products from Spanish date palm (Phoenix dactylifera L., cv. Confitera) co-products for industrial use. Food Chem. 2014, 154, 269–275. [Google Scholar] [CrossRef]
- Chantaro, P.; Devahastin, S.; Chiewchan, N. Production of antioxidant high dietary fiber powder from carrot peels. LWT-Food Sci. Technol. 2008, 41, 1987–1994. [Google Scholar] [CrossRef]
- Tanongkankit, Y.; Chiewchan, N.; Devahastin, S. Physicochemical property changes of cabbage outer leaves upon preparation into functional dietary fiber powder. Food Bioprod. Process. 2012, 90, 541–548. [Google Scholar] [CrossRef]
- Zhong, L.Z.; Fang, Z.X.; Wahlqvist, M.L.; Wu, G.C.; Hodgson, J.M.; Johnson, S.K. Seed coats of pulses as a food ingredient: Characterization, processing, and applications. Trends Food Sci. Technol. 2018, 80, 35–42. [Google Scholar] [CrossRef]
- Ramasamy, U.R.; Gruppen, H.; Kabel, M.A. Water-holding capacity of soluble and insoluble polysaccharides in pressed potato fibre. Ind. Crops Prod. 2015, 64, 242–250. [Google Scholar] [CrossRef]
- Kim, B.R.; Kim, S.; Bae, G.S.; Chang, M.B.; Moon, B. Quality characteristics of common wheat fresh noodle with insoluble dietary fiber from kimchi by-product. LWT-Food Sci. Technol. 2017, 85, 240–245. [Google Scholar] [CrossRef]
- Peerajit, P.; Chiewchan, N.; Devahastin, S. Effects of pretreatment methods on health-related functional properties of high dietary fibre powder from lime residues. Food Chem. 2012, 132, 1891–1898. [Google Scholar] [CrossRef]
- Ramos-Aguilar, O.P.; Ornelas-Paz, J.D.; Ruiz-Cruz, S.; Zamudio-Flores, P.B.; Cervantes-Paz, B.; Gardea-Bejar, A.A.; Perez-Martinez, J.D.; Ibarra-Junquera, V.; Reyes-Hernandez, J. Effect of ripening and heat processing on the physicochemical and rheological properties of pepper pectins. Carbohydr. Polym. 2015, 115, 112–121. [Google Scholar] [CrossRef]
- Benitez, V.; Molla, E.; Martin-Cabrejas, M.A.; Aguilera, Y.; Lopez-Andreu, F.J.; Esteban, R.M. Effect of sterilisation on dietary fibre and physicochemical properties of onion by-products. Food Chem. 2011, 127, 501–507. [Google Scholar] [CrossRef] [Green Version]
- Karaman, E.; Yilmaz, E.; Tuncel, N.B. Physicochemical, microstructural and functional characterization of dietary fibers extracted from lemon, orange and grapefruit seeds press meals. Bioact. Carbohydr. Dietary Fibre 2017, 11, 9–17. [Google Scholar] [CrossRef]
- Sangnark, A.; Noomhorm, A. Effect of particle sizes on functional properties of dietary fibre prepared from sugarcane bagasse. Food Chem. 2003, 80, 221–229. [Google Scholar] [CrossRef]
- Benitez, V.; Molla, E.; Martin-Cabrejas, M.A.; Aguilera, Y.; Esteban, R.M. Physicochemical properties and in vitro antidiabetic potential of fibre concentrates from onion by-products. J. Funct. Foods 2017, 36, 34–42. [Google Scholar] [CrossRef]
- Lan, G.S.; Chen, H.X.; Chen, S.H.; Tian, J.G. Chemical composition and physicochemical properties of dietary fiber from Polygonatum odoratum as affected by different processing methods. Food Res. Int. 2012, 49, 406–410. [Google Scholar] [CrossRef]
- Liu, S.L.; Ni, J.Q.; Radcliffe, J.S.; Vonderohe, C.E. Mitigation of ammonia emissions from pig production using reduced dietary crude protein with amino acid supplementation. Bioresour. Technol. 2017, 233, 200–208. [Google Scholar] [CrossRef]
- Seradj, A.R.; Balcells, J.; Morazan, H.; Alvarez-Rodriguez, J.; Babot, D.; De la Fuente, G. The impact of reducing dietary crude protein and increasing total dietary fiber on hindgut fermentation, the methanogen community and gas emission in growing pigs. Animal Feed Sci. Technol. 2018, 245, 54–66. [Google Scholar] [CrossRef]
- Brownlee, I.A. The physiological roles of dietary fibre. Food Hydrocoll. 2011, 25, 238–250. [Google Scholar] [CrossRef]
- Pan, T.; Xiang, H.Y.; Diao, T.T.; Ma, W.; Shi, C.; Xu, Y.; Xie, Q.H. Effects of probiotics and nutrients addition on the microbial community and fermentation quality of peanut hull. Bioresour. Technol. 2019, 273, 144–152. [Google Scholar] [CrossRef]
- Dowarah, R.; Verma, A.K.; Agarwal, N.; Patel, B.H.M.; Singh, P. Effect of swine based probiotic on performance, diarrhoea scores, intestinal microbiota and gut health of grower-finisher crossbred pigs. Livest. Sci. 2017, 195, 74–79. [Google Scholar] [CrossRef]
- Kosmala, M.; Zdunczyk, Z.; Karlinska, E.; Juskiewicz, J. The effects of strawberry, black currant, and chokeberry extracts in a grain dietary fiber matrix on intestinal fermentation in rats. Food Res. Int. 2014, 64, 752–761. [Google Scholar] [CrossRef]
- Wang, M.; Wichienchot, S.; He, X.; Fu, X.; Huang, Q.; Zhang, B. In vitro colonic fermentation of dietary fibers: Fermentation rate, short-chain fatty acid production and changes in microbiota. Trends Food Sci. Technol. 2019, 88, 1–9. [Google Scholar] [CrossRef]
- Corfield, A.P. Mucins: A biologically relevant glycan barrier in mucosal protection. Biochim. Biophys. Acta Gen. Subj. 2015, 1850, 236–252. [Google Scholar] [CrossRef]
- Fernandez, J.; Redondo-Blanco, S.; Gutierrez-del-Rio, I.; Miguelez, E.M.; Villar, C.J.; Lombo, F. Colon microbiota fermentation of dietary prebiotics towards short-chain fatty acids and their roles as anti-inflammatory and antitumour agents: A review. J. Funct. Foods 2016, 25, 511–522. [Google Scholar] [CrossRef]
- Hamaker, B.R.; Tuncil, Y.E. A perspective on the complexity of dietary fiber structures and their potential effect on the gut microbiota. J. Mol. Biol. 2014, 426, 3838–3850. [Google Scholar] [CrossRef]
- Grzelak-Blaszczyk, K.; Milala, J.; Kosmala, M.; Kolodziejczyk, K.; Sojka, M.; Czarnecki, A.; Klewicki, R.; Juskiewicz, J.; Fotschki, B.; Jurgonski, A. Onion quercetin monoglycosides alter microbial activity and increase antioxidant capacity. J. Nutr. Biochem. 2018, 56, 81–88. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of AOAC International, 16th ed.; AOAC International: Washington, DC, USA, 1995. [Google Scholar]
- Huang, Y.L.; Tsai, Y.H.; Chow, C.J. Water-insoluble fiber-rich fraction from pineapple peel improves intestinal function in hamsters: Evidence from cecal and fecal indicators. Nutr. Res. 2014, 34, 346–354. [Google Scholar] [CrossRef]
- Englyst, H.N.; Quigley, M.E.; Hudson, G.J. Determination of dietary fiber as nonstarch polysaccharides with gas-liquid-chromatographic, high-performance liquid-chromatographic or spectrophotometric measurement of constituent sugars. Analyst 1994, 119, 1497–1509. [Google Scholar] [CrossRef]
- Castillo Andrade, A.I.; Rivera Bautista, C.; Godínez Hernández, C.; Ruiz Cabrera, M.A.; Fuentes Ahumada, C.; García Chávez, E.; Grajales Lagunes, A. Physiometabolic effects of Agave salmiana fructans evaluated in Wistar rats. Int. J. Biol. Macromol. 2018, 108, 1300–1309. [Google Scholar] [CrossRef]
- Goldin, B.R.; Gorbach, S.L. The relationship between diet and rat fecal bacterial enzymes implicated in colon cancer. J. Natl. Cancer Inst. 1976, 57, 371–375. [Google Scholar] [CrossRef]
- Shiau, S.Y.; Chang, G.W. Effects of dietary fiber on fecal mucinase and beta-glucuronidase activity in rats. J. Nutr. 1983, 113, 138–144. [Google Scholar] [CrossRef]
- Okuda, H.; Fujii, S. Kettyuu ammonia tyokusetsu hisyoku triryouhou. Saishin-igaku 1986, 21, 622–627. [Google Scholar]
- Ling, W.H.; Korpela, R.; Mykkanen, H.; Salminen, S.; Hanninen, O. Lactobacillus strain gg supplementation decreases colonic hydrolytic and reductive enzyme-activities in healthy female-adults. J. Nutr. 1994, 124, 18–23. [Google Scholar] [CrossRef]
- Zhu, Z.J.; Zhu, B.W.; Sun, Y.J.; Ai, C.Q.; Wu, S.F.; Wang, L.L.; Song, S.; Liu, X.L. Sulfated polysaccharide from sea cucumber modulates the gut microbiota and its metabolites in normal mice. Int. J. Biol. Macromol. 2018, 120, 502–512. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

| Sample | Protein | Lipid | TDF | Ash | Carbohydrate x |
|---|---|---|---|---|---|
| Unblanched | |||||
| UB-TVSPH | 2.53 ± 0.10 a | 1.10 ± 0.02 a | 71.6 ± 0.02 c | 1.45 ± 0.02 a | 23.3 ± 0.02 a |
| UB-BVSPH | 2.44 ± 0.08 a | 1.09 ± 0.01 a | 71.2 ± 0.01 c | 1.48 ± 0.07 a | 23.8 ± 0.01 a |
| UB-305VSPH | 2.49 ± 0.05 a | 1.07 ± 0.00 a | 75.5 ± 0.02 b | 1.46 ± 0.06 a | 19.5 ± 0.01 b |
| Blanched | |||||
| B-TVSPH | 2.54 ± 0.06 a | 1.09 ± 0.02 a | 74.9 ± 0.01 b | 1.28 ± 0.05 a | 20.2 ± 0.02 b |
| B-BVSPH | 2.41 ± 0.07 a | 1.07 ± 0.01 a | 74.5 ± 0.04 b | 1.26 ± 0.07 a | 20.8 ± 0.01 b |
| B-305VSPH | 2.45 ± 0.05 a | 1.06 ± 0.04 a | 79.0 ± 0.02 a | 1.27 ± 0.04 a | 16.2 ± 0.02 c |
| Sample | WIFF Yield (% Dry wt.) | WIFF | ||
|---|---|---|---|---|
| IDF (% Dry wt.) | SDF (% Dry wt.) | TDF (% Dry wt.) | ||
| Unblanched | ||||
| UB-TVSPH | 69.6 ± 0.21 c | 62.5 ± 0.08 b | 6.27 ± 0.08 d | 68.77 ± 0.15 b |
| UB-BVSPH | 68.4 ± 0.10 c | 62.8 ± 0.15 b | 6.06 ± 0.07 d | 68.86 ± 0.12 b |
| UB-305VSPH | 72.5 ± 0.30 b | 64.9 ± 0.05 a | 8.24 ± 0.08 c | 73.14 ± 0.08 c |
| Blanched | ||||
| B-TVSPH | 72.4 ± 0.02 b | 57.3 ± 0.08 d | 12.5 ± 0.15 b | 69.8 ± 0.08 b |
| B-BVSPH | 71.2 ± 0.10 b | 57.2 ± 0.16 d | 12.1 ± 0.12 b | 69.3 ± 0.11 b |
| B-305VSPH | 74.4 ± 0.32 a | 58.2 ± 0.08 c | 17.5 ± 0.05 a | 75.7 ± 0.05 a |
| WIFF | Rhamnose | Fucose | Arabinose | Xylose | Mannose | Galactose | Glucose | Uronic Acid | Total Sugars |
|---|---|---|---|---|---|---|---|---|---|
| Unblanched | |||||||||
| UB-TVSPH | 0.31 ± 0.01 e | Tr x | 0.88 ± 0.01 d | 15.2 ± 0.10 b | 0.82 ± 0.06 d | 1.46 ± 0.04 d | 30.7 ± 0.20 a | 15.4 ± 0.10 d | 64.8 ± 0.01 c |
| UB-BVSPH | 0.26 ± 0.04 d | Tr | 0.86 ± 0.01 d | 15.1 ± 0.20 b | 0.76 ± 0.03 d | 1.41 ± 0.07 d | 30.5 ± 0.30 a | 14.6 ± 0.04 d | 63.5 ± 0.03 c |
| UB-305VSPH | 0.49 ± 0.01 c | Tr | 1.09 ± 0.01 c | 16.0 ± 0.10 ab | 1.24 ± 0.01 c | 1.85 ± 0.02 c | 32.4 ± 0.15 a | 17.2 ± 0.45 c | 70.3 ± 0.02 b |
| Blanched | |||||||||
| B-TVSPH | 0.69 ± 0.02 b | Tr | 2.43 ± 0.05 b | 17.0 ± 0.10 a | 1.83 ± 0.08 b | 2.78 ± 0.01 b | 26.6 ± 0.15 b | 19.6 ± 0.07 b | 70.9 ± 0.01 b |
| B-BVSPH | 0.65 ± 0.04 b | Tr | 2.40 ± 0.03 b | 16.9 ± 0.21 a | 1.79 ± 0.09 b | 2.73 ± 0.12 b | 26.4 ± 0.25 b | 18.2 ± 0.08 bc | 69.1 ± 0.01 b |
| B-305VSPH | 0.81 ± 0.01 a | Tr | 2.65 ± 0.02 a | 18.0 ± 0.15 a | 2.25 ± 0.02 a | 2.94 ± 0.01 a | 28.4 ± 0.15 b | 20.6 ± 0.11 a | 75.7 ± 0.02 a |
| WIFF | Bulk Density (g/mL) | Water-Retention Capacity (g/g) | Oil-Holding Capacity (g/g) | Swelling Property (mL/g) | Solubility (%) | Cation-Exchange Capacity (meq/kg) |
|---|---|---|---|---|---|---|
| Unblanched | ||||||
| UB-TVSPH | 0.46 ± 0.01 a | 8.12 ± 0.01 d | 5.64 ± 0.03 d | 7.21 ± 0.01 d | 9.29 ± 0.02 c | 187 ± 0.03 d |
| UB-BVSPH | 0.47 ± 0.01 a | 7.77 ± 0.01 e | 5.62 ± 0.01 d | 7.02 ± 0.03 d | 8.17 ± 0.01 d | 175 ± 0.01 e |
| UB-305VSPH | 0.43 ± 0.01 b | 8.53 ± 0.01 c | 6.73 ± 0.02 c | 8.07 ± 0.03 c | 10.1 ± 0.02 b | 201 ± 0.09 b |
| Blanched | ||||||
| B-TVSPH | 0.38 ± 0.01 c | 10.1 ± 0.01 b | 8.28 ± 0.02 b | 9.23 ± 0.02 b | 11.1 ± 0.01 be | 209 ± 0.03 b |
| B-BVSPH | 0.39 ± 0.01 c | 9.62 ± 0.01 b | 8.23 ± 0.02 b | 9.01 ± 0.04 b | 10.5 ± 0.01 b | 196 ± 0.07 c |
| B-305VSPH | 0.34 ± 0.01 d | 11.7 ± 0.01 a | 9.34 ± 0.01 a | 10.8 ± 0.04 a | 12.2 ± 0.01 a | 221 ± 0.02 a |
| Diet | Cecal pH | Cecal Ammonia (μmol/g cecal Content) | Fecal Ammonia (μmol/g Fresh Feces) |
|---|---|---|---|
| Control | 7.30 ± 0.06 a | 4.60 ± 0.06 a | 49.9 ± 0.67 a |
| Cellulose | 6.61 ± 0.02 b | 3.44 ± 0.47 b | 35.1 ± 0.55 b |
| UB-305VSPH WIFF | 6.50 ± 0.06 b | 2.85 ± 0.12 b | 33.2 ± 0.20 b |
| B-305VSPH WIFF | 6.34 ± 0.02 c | 2.39 ± 0.03 c | 28.1 ± 0.17 c |
| Diet | β-d-glucosidase (nmol Nitropheno/min/mg Protein) | β-dglucuronidase (μmol Phenolphthalein/min/mg Protein) | Mucinase (μmol Reducing Sugar/min/mg Protein) | Urease (nmol Ammonia/min/mg Protein) |
|---|---|---|---|---|
| Control | 75.2 ± 0.57 a | 2.14 ± 0.07 a | 0.92 ± 0.02 a | 110 ± 0.40 a |
| Cellulose | 57.9 ± 0.44 b | 1.31 ± 0.01 b | 0.62 ± 0.03 b | 77.5 ± 0.14 b |
| UB-305 VSPH WIFF | 55.4 ± 0.86 b | 1.25 ± 0.03 b | 0.61 ± 0.05 b | 74.9 ± 0.90 b |
| B-305VSPH WIFF | 47.1 ± 0.72 c | 0.89 ± 0.01 c | 0.50 ± 0.02 c | 68.4 ± 0.18 c |
| Diets | Log CFU/g of Wet Feces | |||
|---|---|---|---|---|
| Lactobacillus spp. | Bifidobacterium spp. | Clostridium perfringens | Escherichia coli | |
| Control | 5.20 ± 0.01 c | 5.39 ± 0.01 c | 6.54 ± 0.02 a | 7.39 ± 0.02 a |
| Cellulose | 5.65 ± 0.02 b | 5.84 ± 0.01 b | 6.11 ± 0.01 b | 7.04 ± 0.01 b |
| UB-305VSPH WIFF | 5.79 ± 0.03 b | 5.85 ± 0.03 b | 6.12 ± 0.03 b | 6.95 ± 0.03 b |
| B-305VSPH WIFF | 6.17 ± 0.01 a | 6.27 ± 0.02 a | 5.84 ± 0.01 c | 6.60 ± 0.03 c |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.-L.; Hsieh, I.-T. Physicochemical Properties and Intestinal Health Promoting Water-Insoluble Fiber Enriched Fraction Prepared from Blanched Vegetable Soybean Pod Hulls. Molecules 2019, 24, 1796. https://doi.org/10.3390/molecules24091796
Huang Y-L, Hsieh I-T. Physicochemical Properties and Intestinal Health Promoting Water-Insoluble Fiber Enriched Fraction Prepared from Blanched Vegetable Soybean Pod Hulls. Molecules. 2019; 24(9):1796. https://doi.org/10.3390/molecules24091796
Chicago/Turabian StyleHuang, Ya-Ling, and I-Ting Hsieh. 2019. "Physicochemical Properties and Intestinal Health Promoting Water-Insoluble Fiber Enriched Fraction Prepared from Blanched Vegetable Soybean Pod Hulls" Molecules 24, no. 9: 1796. https://doi.org/10.3390/molecules24091796
APA StyleHuang, Y. -L., & Hsieh, I. -T. (2019). Physicochemical Properties and Intestinal Health Promoting Water-Insoluble Fiber Enriched Fraction Prepared from Blanched Vegetable Soybean Pod Hulls. Molecules, 24(9), 1796. https://doi.org/10.3390/molecules24091796