β-Caryophyllene, a CB2-Receptor-Selective Phytocannabinoid, Suppresses Mechanical Allodynia in a Mouse Model of Antiretroviral-Induced Neuropathic Pain
Abstract
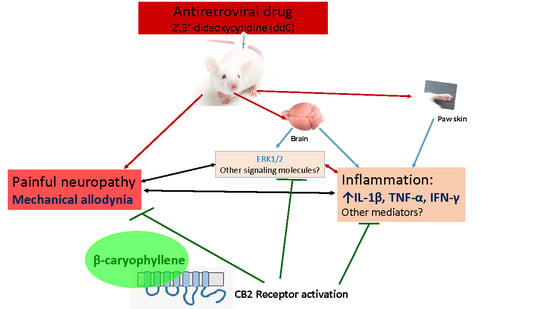
:1. Introduction
2. Results
2.1. Effect of ddC on Withdrawal Threshold to Mechanical and Thermal Stimulation
2.2. β-Caryophyllene, Minocycline and Pentoxifylline Prevent the Development of ddC-Induced Mechanical Allodynia
2.3. β-Caryophyllene Attenuates Established ddC-Induced Mechanical Allodynia in a CB2-Receptor-Dependent Manner
2.4. β-Caryophyllene Prevents the ddC-Induced Upregulation of Proinflammatory Cytokine Transcripts in the Paw Skin and Brain
2.5. ddC Does Not Alter Glial Cell Markers in the Brain
2.6. β-Caryophyllene Prevents the ddC-Induced Phospho-Erk1/2 Levels in the Brain
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Drugs
4.3. Model of ddC-Induced Neuropathic Pain and Drug Treatment
4.4. Assessment of Mechanical Allodynia
4.5. Assessment of Response to Thermal Stimuli
4.6. Disecction and Tissue Storage
4.7. Real Time RT-PCR
4.8. Western Blot
4.8.1. WesTM Capillary-Based Protein Electrophoresis
4.8.2. Gel-Based Western Blot
4.9. Statistical Analyses
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Carpenter, C.C.; Fischl, M.A.; Hammer, S.M.; Hirsch, M.S.; Jacobsen, D.M.; Katzenstein, D.A.; Montaner, J.S.; Richman, D.D.; Saag, M.S.; Schooley, R.T.; et al. Antiretroviral therapy for HIV infection in 1996. Recommendations of an international panel. International AIDS Society-USA. Jama 1996, 276, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Vella, S.; Schwartlander, B.; Sow, S.P.; Eholie, S.P.; Murphy, R.L. The history of antiretroviral therapy and of its implementation in resource-limited areas of the world. Aids 2012, 26, 1231–1241. [Google Scholar] [CrossRef] [PubMed]
- Pau, A.K.; George, J.M. Antiretroviral therapy: Current drugs. Infect. Dis. Clin. N. Am. 2014, 28, 371–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunthard, H.F.; Saag, M.S.; Benson, C.A.; del Rio, C.; Eron, J.J.; Gallant, J.E.; Hoy, J.F.; Mugavero, M.J.; Sax, P.E.; Thompson, M.A.; et al. Antiretroviral Drugs for Treatment and Prevention of HIV Infection in Adults: 2016 Recommendations of the International Antiviral Society-USA Panel. Jama 2016, 316, 191–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cihlar, T.; Ray, A.S. Nucleoside and nucleotide HIV reverse transcriptase inhibitors: 25 years after zidovudine. Antivir. Res. 2010, 85, 39–58. [Google Scholar] [CrossRef]
- Stavros, K.; Simpson, D.M. Understanding the etiology and management of HIV-associated peripheral neuropathy. Curr. HIV/AIDS Rep. 2014, 11, 195–201. [Google Scholar] [CrossRef]
- Dalakas, M.C. Peripheral neuropathy and antiretroviral drugs. J. Peripher. Nerv. Syst. 2001, 6, 14–20. [Google Scholar] [CrossRef]
- Kamerman, P.R.; Moss, P.J.; Weber, J.; Wallace, V.C.; Rice, A.S.; Huang, W. Pathogenesis of HIV-associated sensory neuropathy: Evidence from in vivo and in vitro experimental models. J. Peripher. Nerv. Syst. 2012, 17, 19–31. [Google Scholar] [CrossRef]
- Schutz, S.G.; Robinson-Papp, J. HIV-related neuropathy: Current perspectives. Hiv/Aids 2013, 5, 243–251. [Google Scholar]
- Hao, S. The molecular and pharmacological mechanisms of hiv-related neuropathic pain. Curr. Neuropharmacol. 2013, 11, 499–512. [Google Scholar] [CrossRef] [Green Version]
- Joseph, E.K.; Chen, X.; Khasar, S.G.; Levine, J.D. Novel mechanism of enhanced nociception in a model of AIDS therapy-induced painful peripheral neuropathy in the rat. Pain 2004, 107, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Munawar, N.; Oriowo, M.A.; Masocha, W. Antihyperalgesic Activities of Endocannabinoids in a Mouse Model of Antiretroviral-Induced Neuropathic Pain. Front. Pharmacol. 2017, 8, 136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Levine, J.D. Mechanically-evoked C-fiber activity in painful alcohol and AIDS therapy neuropathy in the rat. Mol. Pain 2007, 3, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, W.; Zheng, W.; Ouyang, H.; Yi, H.; Liu, S.; Zeng, W.; Levitt, R.C.; Candiotti, K.A.; Lubarsky, D.A.; Hao, S. Mechanical allodynia induced by nucleoside reverse transcriptase inhibitor is suppressed by p55TNFSR mediated by herpes simplex virus vector through the SDF1alpha/CXCR4 system in rats. Anesth. Analg. 2014, 118, 671–680. [Google Scholar] [CrossRef]
- Bhangoo, S.K.; Ren, D.; Miller, R.J.; Chan, D.M.; Ripsch, M.S.; Weiss, C.; McGinnis, C.; White, F.A. CXCR4 chemokine receptor signaling mediates pain hypersensitivity in association with antiretroviral toxic neuropathy. Brain Behav. Immun. 2007, 21, 581–591. [Google Scholar] [CrossRef] [Green Version]
- Joseph, E.K.; Levine, J.D. Caspase signalling in neuropathic and inflammatory pain in the rat. Eur. J. Neurosci. 2004, 20, 2896–2902. [Google Scholar] [CrossRef]
- Yuan, S.; Shi, Y.; Guo, K.; Tang, S.J. Nucleoside Reverse Transcriptase Inhibitors (NRTIs) Induce Pathological Pain through Wnt5a-Mediated Neuroinflammation in Aging Mice. J. Neuroimmune Pharmacol. 2018, 13, 230–236. [Google Scholar] [CrossRef]
- Ferrari, L.F.; Chum, A.; Bogen, O.; Reichling, D.B.; Levine, J.D. Role of Drp1, a key mitochondrial fission protein, in neuropathic pain. J. Neurosci. 2011, 31, 11404–11410. [Google Scholar] [CrossRef]
- Joseph, E.K.; Levine, J.D. Mitochondrial electron transport in models of neuropathic and inflammatory pain. Pain 2006, 121, 105–114. [Google Scholar] [CrossRef]
- Keilbaugh, S.A.; Prusoff, W.H.; Simpson, M.V. The PC12 cell as a model for studies of the mechanism of induction of peripheral neuropathy by anti-HIV-1 dideoxynucleoside analogs. Biochem. Pharmacol. 1991, 42, R5–R8. [Google Scholar] [CrossRef]
- Kaku, M.; Simpson, D.M. HIV neuropathy. Curr. Opin. HIV AIDS 2014, 9, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Kieburtz, K.; Simpson, D.; Yiannoutsos, C.; Max, M.B.; Hall, C.D.; Ellis, R.J.; Marra, C.M.; McKendall, R.; Singer, E.; Dal Pan, G.J.; et al. A randomized trial of amitriptyline and mexiletine for painful neuropathy in HIV infection. AIDS Clinical Trial Group 242 Protocol Team. Neurology 1998, 51, 1682–1688. [Google Scholar] [CrossRef] [PubMed]
- Shlay, J.C.; Chaloner, K.; Max, M.B.; Flaws, B.; Reichelderfer, P.; Wentworth, D.; Hillman, S.; Brizz, B.; Cohn, D.L. Acupuncture and amitriptyline for pain due to HIV-related peripheral neuropathy: A randomized controlled trial. Terry Beirn Community Programs for Clinical Research on AIDS. Jama 1998, 280, 1590–1595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hahn, K.; Arendt, G.; Braun, J.S.; von Giesen, H.J.; Husstedt, I.W.; Maschke, M.; Straube, M.E.; Schielke, E.; German Neuro, A.W.G. A placebo-controlled trial of gabapentin for painful HIV-associated sensory neuropathies. J. Neurol. 2004, 251, 1260–1266. [Google Scholar] [CrossRef] [PubMed]
- Simpson, D.M.; Schifitto, G.; Clifford, D.B.; Murphy, T.K.; Durso-De Cruz, E.; Glue, P.; Whalen, E.; Emir, B.; Scott, G.N.; Freeman, R.; et al. Pregabalin for painful HIV neuropathy: A randomized, double-blind, placebo-controlled trial. Neurology 2010, 74, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Duarte, A.; Robinson-Papp, J.; Simpson, D.M. Diagnosis and management of HIV-associated neuropathy. Neurol. Clin. 2008, 26, 821–832. [Google Scholar] [CrossRef]
- Phillips, T.J.; Cherry, C.L.; Cox, S.; Marshall, S.J.; Rice, A.S. Pharmacological treatment of painful HIV-associated sensory neuropathy: A systematic review and meta-analysis of randomised controlled trials. PLoS ONE 2010, 5, e14433. [Google Scholar] [CrossRef]
- Abrams, D.I.; Jay, C.A.; Shade, S.B.; Vizoso, H.; Reda, H.; Press, S.; Kelly, M.E.; Rowbotham, M.C.; Petersen, K.L. Cannabis in painful HIV-associated sensory neuropathy: A randomized placebo-controlled trial. Neurology 2007, 68, 515–521. [Google Scholar] [CrossRef]
- Ellis, R.J.; Toperoff, W.; Vaida, F.; van den Brande, G.; Gonzales, J.; Gouaux, B.; Bentley, H.; Atkinson, J.H. Smoked medicinal cannabis for neuropathic pain in HIV: A randomized, crossover clinical trial. Neuropsychopharmacology 2009, 34, 672–680. [Google Scholar] [CrossRef] [Green Version]
- Simpson, D.M.; Brown, S.; Tobias, J.; Group, N.-C.S. Controlled trial of high-concentration capsaicin patch for treatment of painful HIV neuropathy. Neurology 2008, 70, 2305–2313. [Google Scholar] [CrossRef]
- Meacher, B.M. Against the stream: Drugs policy needs to be turned on its head. BJPsych Bull. 2018, 43, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Rice, A.S. Should cannabinoids be used as analgesics for neuropathic pain? Nat. Rev. Neurol. 2008, 4, 654–655. [Google Scholar] [CrossRef] [PubMed]
- Gertsch, J.; Leonti, M.; Raduner, S.; Racz, I.; Chen, J.Z.; Xie, X.Q.; Altmann, K.H.; Karsak, M.; Zimmer, A. Beta-caryophyllene is a dietary cannabinoid. Proc. Natl. Acad. Sci. USA 2008, 105, 9099–9104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russo, E.B. Beyond Cannabis: Plants and the Endocannabinoid System. Trends Pharmacol. Sci. 2016, 37, 594–605. [Google Scholar] [CrossRef]
- Rahn, E.J.; Hohmann, A.G. Cannabinoids as pharmacotherapies for neuropathic pain: From the bench to the bedside. Neurotherapeutics 2009, 6, 713–737. [Google Scholar] [CrossRef]
- Klauke, A.L.; Racz, I.; Pradier, B.; Markert, A.; Zimmer, A.M.; Gertsch, J.; Zimmer, A. The cannabinoid CB2 receptor-selective phytocannabinoid beta-caryophyllene exerts analgesic effects in mouse models of inflammatory and neuropathic pain. Eur. Neuropsychopharmacol. 2014, 24, 608–620. [Google Scholar] [CrossRef] [Green Version]
- Poddighe, L.; Carta, G.; Serra, M.P.; Melis, T.; Boi, M.; Lisai, S.; Murru, E.; Muredda, L.; Collu, M.; Banni, S.; et al. Acute administration of beta-caryophyllene prevents endocannabinoid system activation during transient common carotid artery occlusion and reperfusion. Lipids Health Dis. 2018, 17, 23. [Google Scholar] [CrossRef] [Green Version]
- Dahham, S.; Tabana, Y.; Ahamed, M.B.K.; Abdul Majid, A.M.S. In vivo anti-inflammatory activity of β-caryophyllene, evaluated by molecular imaging. Mol. Med. Chem. 2015, 1, e1001. [Google Scholar]
- Alberti, T.B.; Barbosa, W.L.; Vieira, J.L.; Raposo, N.R.; Dutra, R.C. (−)-beta-Caryophyllene, a CB2 receptor-selective phytocannabinoid, suppresses motor paralysis and neuroinflammation in a murine model of multiple sclerosis. Int. J. Mol. Sci. 2017, 18, 691. [Google Scholar] [CrossRef]
- Galdino, P.M.; Nascimento, M.V.M.; Florentino, I.F.; Lino, R.C.; Fajemiroye, J.O.; Chaibub, B.A.; de Paula, J.R.; de Lima, T.C.M.; Costa, E.A. The anxiolytic-like effect of an essential oil derived from Spiranthera odoratissima A. St. Hil. leaves and its major component, β-caryophyllene, in male mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2012, 38, 276–284. [Google Scholar] [CrossRef] [Green Version]
- Fidyt, K.; Fiedorowicz, A.; Strzadala, L.; Szumny, A. beta-caryophyllene and beta-caryophyllene oxide-natural compounds of anticancer and analgesic properties. Cancer Med. 2016, 5, 3007–3017. [Google Scholar] [CrossRef] [PubMed]
- Segat, G.C.; Manjavachi, M.N.; Matias, D.O.; Passos, G.F.; Freitas, C.S.; Costa, R.; Calixto, J.B. Antiallodynic effect of beta-caryophyllene on paclitaxel-induced peripheral neuropathy in mice. Neuropharmacology 2017, 125, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Ávila, D.S.; Flores-Soto, M.E.; Tapia-Vázquez, C.; Pastor-Zarandona, O.A.; López-Roa, R.I.; Viveros-Paredes, J.M. β-Caryophyllene, a Natural Sesquiterpene, Attenuates Neuropathic Pain and Depressive-Like Behavior in Experimental Diabetic Mice. J. Med. Food 2019, 22, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Paula-Freire, L.I.; Andersen, M.L.; Gama, V.S.; Molska, G.R.; Carlini, E.L. The oral administration of trans-caryophyllene attenuates acute and chronic pain in mice. Phytomedicine 2014, 21, 356–362. [Google Scholar] [CrossRef]
- Kuwahata, H. Local Peripheral Effects of β -Caryophyllene through CB2 Receptors in Neuropathic Pain in Mice. Pharmacol. Pharm. 2012, 3, 397–403. [Google Scholar] [CrossRef] [Green Version]
- Guasti, L.; Richardson, D.; Jhaveri, M.; Eldeeb, K.; Barrett, D.; Elphick, M.R.; Alexander, S.P.; Kendall, D.; Michael, G.J.; Chapman, V. Minocycline treatment inhibits microglial activation and alters spinal levels of endocannabinoids in a rat model of neuropathic pain. Mol. Pain 2009, 5, 35. [Google Scholar] [CrossRef]
- Kim, H.K.; Hwang, S.H.; Lee, S.O.; Kim, S.H.; Abdi, S. Pentoxifylline Ameliorates Mechanical Hyperalgesia in a Rat Model of Chemotherapy-Induced Neuropathic Pain. Pain Physician 2016, 19, E589–E600. [Google Scholar]
- Liu, J.; Feng, X.; Yu, M.; Xie, W.; Zhao, X.; Li, W.; Guan, R.; Xu, J. Pentoxifylline attenuates the development of hyperalgesia in a rat model of neuropathic pain. Neurosci. Lett. 2007, 412, 268–272. [Google Scholar] [CrossRef]
- Padi, S.S.; Kulkarni, S.K. Minocycline prevents the development of neuropathic pain, but not acute pain: Possible anti-inflammatory and antioxidant mechanisms. Eur. J. Pharmacol. 2008, 601, 79–87. [Google Scholar] [CrossRef]
- Masocha, W.; Thomas, A. Indomethacin plus minocycline coadministration relieves chemotherapy and antiretroviral drug-induced neuropathic pain in a cannabinoid receptors-dependent manner. J. Pharmacol. Sci. 2019, 139, 325–332. [Google Scholar] [CrossRef]
- Sanna, M.D.; Quattrone, A.; Ghelardini, C.; Galeotti, N. PKC-mediated HuD-GAP43 pathway activation in a mouse model of antiretroviral painful neuropathy. Pharmacol. Res. 2014, 81, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Bouhassira, D.; Attal, N.; Willer, J.C.; Brasseur, L. Painful and painless peripheral sensory neuropathies due to HIV infection: A comparison using quantitative sensory evaluation. Pain 1999, 80, 265–272. [Google Scholar] [CrossRef]
- Martin, C.; Solders, G.; Sonnerborg, A.; Hansson, P. Painful and non-painful neuropathy in HIV-infected patients: An analysis of somatosensory nerve function. Eur. J. Pain 2003, 7, 23–31. [Google Scholar] [CrossRef]
- Wallace, V.C.J.; Blackbeard, J.; Segerdahl, A.R.; Hasnie, F.; Pheby, T.; McMahon, S.B.; Rice, A.S.C. Characterization of rodent models of HIV-gp120 and anti-retroviral-associated neuropathic pain. Brain 2007, 130 Pt 10, 2688–2702. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.; Ouyang, H.; Liu, S.; Mata, M.; Fink, D.J.; Hao, S. TNFα is involved in neuropathic pain induced by nucleoside reverse transcriptase inhibitor in rats. Brain Behav. Immun. 2011, 25, 1668–1676. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Zhang, Y.-Q.; Qadri, Y.J.; Serhan, C.N.; Ji, R.-R. Microglia in Pain: Detrimental and Protective Roles in Pathogenesis and Resolution of Pain. Neuron 2018, 100, 1292–1311. [Google Scholar] [CrossRef] [Green Version]
- Dance, A. Why the sexes don’t feel pain the same way. Nature 2019, 567, 448–450. [Google Scholar] [CrossRef] [Green Version]
- Sorge, R.E.; Mapplebeck, J.C.; Rosen, S.; Beggs, S.; Taves, S.; Alexander, J.K.; Martin, L.J.; Austin, J.S.; Sotocinal, S.G.; Chen, D.; et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat. Neurosci. 2015, 18, 1081–1083. [Google Scholar] [CrossRef] [Green Version]
- Ebersberger, A. The analgesic potential of cytokine neutralization with biologicals. Eur. J. Pharmacol. 2018, 835, 19–30. [Google Scholar] [CrossRef]
- Hung, A.L.; Lim, M.; Doshi, T.L. Targeting cytokines for treatment of neuropathic pain. Scand. J. Pain 2017, 17, 287–293. [Google Scholar] [CrossRef]
- Racz, I.; Nadal, X.; Alferink, J.; Banos, J.E.; Rehnelt, J.; Martin, M.; Pintado, B.; Gutierrez-Adan, A.; Sanguino, E.; Bellora, N.; et al. Interferon-gamma is a critical modulator of CB2 cannabinoid receptor signaling during neuropathic pain. J. Neurosci. 2008, 28, 12136–12145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robertson, B.; Xu, X.J.; Hao, J.X.; Wiesenfeld-Hallin, Z.; Mhlanga, J.; Grant, G.; Kristensson, K. Interferon-gamma receptors in nociceptive pathways: Role in neuropathic pain-related behaviour. Neuroreport 1997, 8, 1311–1316. [Google Scholar] [CrossRef] [PubMed]
- Schafers, M.; Brinkhoff, J.; Neukirchen, S.; Marziniak, M.; Sommer, C. Combined epineurial therapy with neutralizing antibodies to tumor necrosis factor-alpha and interleukin-1 receptor has an additive effect in reducing neuropathic pain in mice. Neurosci. Lett. 2001, 310, 113–116. [Google Scholar] [CrossRef]
- Shubayev, V.I.; Myers, R.R. Upregulation and interaction of TNFalpha and gelatinases A and B in painful peripheral nerve injury. Brain Res. 2000, 855, 83–89. [Google Scholar] [CrossRef]
- Sommer, C.; Leinders, M.; Uceyler, N. Inflammation in the pathophysiology of neuropathic pain. Pain 2018, 159, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Van der Watt, J.J.; Wilkinson, K.A.; Wilkinson, R.J.; Heckmann, J.M. Plasma cytokine profiles in HIV-1 infected patients developing neuropathic symptoms shortly after commencing antiretroviral therapy: A case-control study. BMC Infect. Dis. 2014, 14, 71. [Google Scholar] [CrossRef] [Green Version]
- Ji, R.R.; Suter, M.R. p38 MAPK, microglial signaling, and neuropathic pain. Mol. Pain 2007, 3, 33. [Google Scholar] [CrossRef] [Green Version]
- Luo, X.; Wang, X.; Xia, Z.; Chung, S.K.; Cheung, C.W. CXCL12/CXCR4 axis: An emerging neuromodulator in pathological pain. Rev. Neurosci. 2016, 27, 83–92. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Calvo, M.; Karu, K.; Olausen, H.R.; Bathgate, G.; Okuse, K.; Bennett, D.L.; Rice, A.S. A clinically relevant rodent model of the HIV antiretroviral drug stavudine induced painful peripheral neuropathy. Pain 2013, 154, 560–575. [Google Scholar] [CrossRef]
- Zheng, W.; Huang, W.; Liu, S.; Levitt, R.C.; Candiotti, K.A.; Lubarsky, D.A.; Hao, S. IL-10 mediated by herpes simplex virus vector reduces neuropathic pain induced by HIV gp120 combined with ddC in rats. Mol. Pain 2014, 10, 49. [Google Scholar] [CrossRef] [Green Version]
- Maruta, T.; Nemoto, T.; Hidaka, K.; Koshida, T.; Shirasaka, T.; Yanagita, T.; Takeya, R.; Tsuneyoshi, I. Upregulation of ERK phosphorylation in rat dorsal root ganglion neurons contributes to oxaliplatin-induced chronic neuropathic pain. PLoS ONE 2019, 14, e0225586. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.; Ouyang, S.; Zhou, C.; Xie, L.; Fang, Z.; Yuan, H.; Yang, J.; Zou, L.; Jia, T.; Zhao, S.; et al. Andrographolide Inhibits Mechanical and Thermal Hyperalgesia in a Rat Model of HIV-Induced Neuropathic Pain. Front. Pharmacol. 2018, 9, 593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bento, A.F.; Marcon, R.; Dutra, R.C.; Claudino, R.F.; Cola, M.; Leite, D.F.; Calixto, J.B. beta-Caryophyllene inhibits dextran sulfate sodium-induced colitis in mice through CB2 receptor activation and PPARgamma pathway. Am. J. Pathol. 2011, 178, 1153–1166. [Google Scholar] [CrossRef] [PubMed]
- Munro, S.; Thomas, K.L.; Abu-Shaar, M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993, 365, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.M.; Stella, N. CB2 receptor-mediated migration of immune cells: It can go either way. Br. J. Pharmacol. 2008, 153, 299–308. [Google Scholar] [CrossRef] [Green Version]
- Rom, S.; Persidsky, Y. Cannabinoid receptor 2: Potential role in immunomodulation and neuroinflammation. J. Neuroimmune Pharmacol. 2013, 8, 608–620. [Google Scholar] [CrossRef] [Green Version]
- Turcotte, C.; Blanchet, M.R.; Laviolette, M.; Flamand, N. The CB2 receptor and its role as a regulator of inflammation. Cell. Mol. Life Sci. 2016, 73, 4449–4470. [Google Scholar] [CrossRef] [Green Version]
- Parvathy, S.S.; Masocha, W. Coadministration of indomethacin and minocycline attenuates established paclitaxel-induced neuropathic thermal hyperalgesia: Involvement of cannabinoid CB1 receptors. Sci. Rep. 2015, 5, 10541. [Google Scholar] [CrossRef] [Green Version]
- Zarei, M.; Sabetkasaei, M.; Moini-Zanjani, T. Paradoxical effect of minocycline on established neuropathic pain in rat. EXCLI J. 2017, 16, 229–235. [Google Scholar]
- Ji, X.T.; Qian, N.S.; Zhang, T.; Li, J.M.; Li, X.K.; Wang, P.; Zhao, D.S.; Huang, G.; Zhang, L.; Fei, Z.; et al. Spinal astrocytic activation contributes to mechanical allodynia in a rat chemotherapy-induced neuropathic pain model. PLoS ONE 2013, 8, e60733. [Google Scholar] [CrossRef] [Green Version]
- Sumitani, M.; Ueda, H.; Hozumi, J.; Inoue, R.; Kogure, T.; Yamada, Y.; Kogure, T. Minocycline Does Not Decrease Intensity of Neuropathic Pain Intensity, But Does Improve Its Affective Dimension. J. Pain Palliat. Care Pharmacother. 2016, 30, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Zafra, T.; Gao, T.; Jurczak, A.; Sandor, K.; Hore, Z.; Agalave, N.M.; Su, J.; Estelius, J.; Lampa, J.; Hokfelt, T.; et al. Exploring the transcriptome of resident spinal microglia after collagen antibody-induced arthritis. Pain 2019, 160, 224–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Cesare Mannelli, L.; Cinci, L.; Micheli, L.; Zanardelli, M.; Pacini, A.; McIntosh, J.M.; Ghelardini, C. α-conotoxin RgIA protects against the development of nerve injury-induced chronic pain and prevents both neuronal and glial derangement. Pain 2014, 155, 1986–1995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mika, J.; Osikowicz, M.; Rojewska, E.; Korostynski, M.; Wawrzczak-Bargiela, A.; Przewlocki, R.; Przewlocka, B. Differential activation of spinal microglial and astroglial cells in a mouse model of peripheral neuropathic pain. Eur. J. Pharmacol. 2009, 623, 65–72. [Google Scholar] [CrossRef]
- Zhang, H.; Yoon, S.-Y.; Zhang, H.; Dougherty, P.M. Evidence that spinal astrocytes but not microglia contribute to the pathogenesis of Paclitaxel-induced painful neuropathy. J. Pain 2012, 13, 293–303. [Google Scholar] [CrossRef] [Green Version]
- Garcia, F.A.; Reboucas, J.F.; Balbino, T.Q.; da Silva, T.G.; de Carvalho-Junior, C.H.; Cerqueira, G.S.; Brito, G.A.; Viana, G.S. Pentoxifylline reduces the inflammatory process in diabetic rats: Relationship with decreases of pro-inflammatory cytokines and inducible nitric oxide synthase. J. Inflamm. 2015, 12, 33. [Google Scholar] [CrossRef] [Green Version]
- Nowak, L.; Zurowski, D.; Dobrogowski, J.; Wordliczek, J.; Thor, P.J. Pentoxifylline modifies central and peripheral vagal mechanism in acute and chronic pain models. Folia Med. Crac. 2012, 52, 83–95. [Google Scholar]
- Ledeboer, A.; Sloane, E.M.; Milligan, E.D.; Frank, M.G.; Mahony, J.H.; Maier, S.F.; Watkins, L.R. Minocycline attenuates mechanical allodynia and proinflammatory cytokine expression in rat models of pain facilitation. Pain 2005, 115, 71–83. [Google Scholar] [CrossRef]
- Pabreja, K.; Dua, K.; Sharma, S.; Padi, S.S.; Kulkarni, S.K. Minocycline attenuates the development of diabetic neuropathic pain: Possible anti-inflammatory and anti-oxidant mechanisms. Eur. J. Pharmacol. 2011, 661, 15–21. [Google Scholar] [CrossRef]
- Raghavendra, V.; Tanga, F.; DeLeo, J.A. Inhibition of microglial activation attenuates the development but not existing hypersensitivity in a rat model of neuropathy. J. Pharmacol. Exp. Ther. 2003, 306, 624–630. [Google Scholar] [CrossRef] [Green Version]
- Mika, J.; Osikowicz, M.; Makuch, W.; Przewlocka, B. Minocycline and pentoxifylline attenuate allodynia and hyperalgesia and potentiate the effects of morphine in rat and mouse models of neuropathic pain. Eur. J. Pharmacol. 2007, 560, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Masocha, W.; Parvathy, S.S. Preventative and therapeutic effects of a GABA transporter 1 inhibitor administered systemically in a mouse model of paclitaxel-induced neuropathic pain. PeerJ 2016, 4, e2798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parvathy, S.S.; Masocha, W. Matrix metalloproteinase inhibitor COL-3 prevents the development of paclitaxel-induced hyperalgesia in mice. Med Princ. Pract. 2013, 22, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Masocha, W. Systemic lipopolysaccharide (LPS)-induced microglial activation results in different temporal reduction of CD200 and CD200 receptor gene expression in the brain. J. Neuroimmunol. 2009, 214, 78–82. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- El-Tabba, R.M.; Mathew, P.; Masocha, W.; Khajah, M.A. COL3 enhances the antiproliferative and proapoptotic effects of paclitaxel in breast cancer cells. Oncol. Rep. 2019, 41, 630–642. [Google Scholar]








| Gene | Polarity | |
|---|---|---|
| Sense Sequence 5′–3′ | Anti-Sense Sequence 5′–3′ | |
| Ppia (cyclophilin A) | GCTTTTCGCCGCTTGCT | CTCGTCATCGGCCGTGAT |
| Ifng (interferon gamma) | ACAATGAACGCTACACACTGCAT | TGGCAGTAACAGCCAGAAACA |
| Il1b (interleukin 1 beta | TGGTGTGTGACGTTCCCATT | CAGCACGAGGCTTTTTTGTTG |
| Tnf (tumor necrosis factor alpha) | GGCTGCCCCGACTACGT | GACTTTCTCCTGGTATGAGATAGCAAA |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aly, E.; Khajah, M.A.; Masocha, W. β-Caryophyllene, a CB2-Receptor-Selective Phytocannabinoid, Suppresses Mechanical Allodynia in a Mouse Model of Antiretroviral-Induced Neuropathic Pain. Molecules 2020, 25, 106. https://doi.org/10.3390/molecules25010106
Aly E, Khajah MA, Masocha W. β-Caryophyllene, a CB2-Receptor-Selective Phytocannabinoid, Suppresses Mechanical Allodynia in a Mouse Model of Antiretroviral-Induced Neuropathic Pain. Molecules. 2020; 25(1):106. https://doi.org/10.3390/molecules25010106
Chicago/Turabian StyleAly, Esraa, Maitham A. Khajah, and Willias Masocha. 2020. "β-Caryophyllene, a CB2-Receptor-Selective Phytocannabinoid, Suppresses Mechanical Allodynia in a Mouse Model of Antiretroviral-Induced Neuropathic Pain" Molecules 25, no. 1: 106. https://doi.org/10.3390/molecules25010106
APA StyleAly, E., Khajah, M. A., & Masocha, W. (2020). β-Caryophyllene, a CB2-Receptor-Selective Phytocannabinoid, Suppresses Mechanical Allodynia in a Mouse Model of Antiretroviral-Induced Neuropathic Pain. Molecules, 25(1), 106. https://doi.org/10.3390/molecules25010106