Lipoteichoic Acid Biosynthesis Inhibitors as Potent Inhibitors of S. aureus and E. faecalis Growth and Biofilm Formation
Abstract
:1. Introduction
2. Results and Discussion
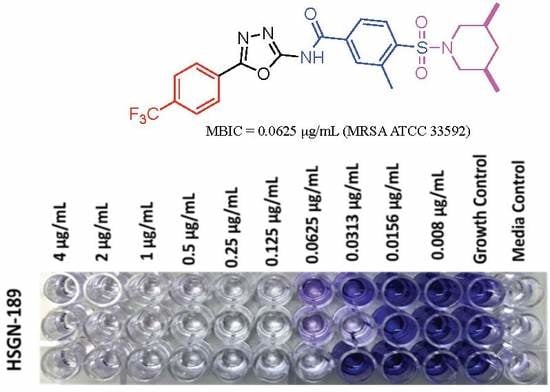
2.1. Biofilm Inhibition Activity of HSGN-94 and HSGN-189 against MRSA and VRE Strains
2.2. HSGN-94 and HSGN-189 Synergize with Tunicamycin and Targocil against MRSA and VRE Strains
2.3. HSGN-94 and HSGN-189 Shows Synergy with Tunicamycin in Inhibiting MRSA and VRE Biofilms
3. Materials and Methods
3.1. Bacterial Strains and Chemical Compounds
3.2. Synergistic Interactions of HSGN-94 and HSGN-189 with Tunicamycin and Targocil
3.3. Biofilm Inhibition Assay and Minimum Biofilm Inhibition Concentration (MBIC)
3.4. Biofilm Eradication Assay and Minimum Biofilm Eradication Concentration (MBEC)
3.5. MBIC Synergy with Tunicamycin
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- de Kraker, M.E.; Stewardson, A.J.; Harbarth, S. Will 10 Million People Die a Year due to Antimicrobial Resistance by 2050? PLoS Med. 2016, 13, e1002184. [Google Scholar] [CrossRef] [Green Version]
- Frieden, T.; Centers for Disease Control and Prevention (CDC). Antibiotic Resistance Threats in the United States. 2013. Available online: https://www.cdc.gov/drugresistance/threat-report-2013/ (accessed on 2 March 2020).
- Mah, T.F.; O’Toole, G.A. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001, 9, 34–39. [Google Scholar] [CrossRef]
- Ciofu, O.; Rojo-Molinero, E.; Macia, M.D.; Oliver, A. Antibiotic treatment of biofilm infections. APMIS 2017, 125, 304–319. [Google Scholar] [CrossRef]
- Rajagopal, M.; Walker, S. Envelope Structures of Gram-Positive Bacteria. Curr. Top. Microbiol. Immunol. 2017, 404, 1–44. [Google Scholar]
- Brown, S.; Santa Maria, J.P., Jr.; Walker, S. Wall teichoic acids of gram-positive bacteria. Annu. Rev. Microbiol. 2013, 67, 313–336. [Google Scholar] [CrossRef] [Green Version]
- Swoboda, J.G.; Campbell, J.; Meredith, T.C.; Walker, S. Wall teichoic acid function, biosynthesis, and inhibition. Chembiochem 2010, 11, 35–45. [Google Scholar] [CrossRef] [Green Version]
- Percy, M.G.; Gründling, A. Lipoteichoic Acid Synthesis and Function in Gram-Positive Bacteria. Annu. Rev. Microbiol. 2014, 68, 81–100. [Google Scholar] [CrossRef] [Green Version]
- Gross, M.; Cramton, S.E.; Gotz, F.; Peschel, A. Key role of teichoic acid net charge in Staphylococcus aureus colonization of artificial surfaces. Infect. Immun. 2001, 69, 3423–3426. [Google Scholar] [CrossRef] [Green Version]
- Fabretti, F.; Theilacker, C.; Baldassarri, L.; Kaczynski, Z.; Kropec, A.; Holst, O.; Huebner, J. Alanine esters of enterococcal lipoteichoic acid play a role in biofilm formation and resistance to antimicrobial peptides. Infect. Immun. 2006, 74, 4164–4171. [Google Scholar] [CrossRef] [Green Version]
- Weidenmaier, C.; Kokai-Kun, J.F.; Kristian, S.A.; Chanturiya, T.; Kalbacher, H.; Gross, M.; Nicholson, G.; Neumeister, B.; Mond, J.J.; Peschel, A. Role of teichoic acids in Staphylococcus aureus nasal colonization, a major risk factor in nosocomial infections. Nat. Med. 2004, 10, 243–245. [Google Scholar] [CrossRef]
- Fedtke, I.; Mader, D.; Kohler, T.; Moll, H.; Nicholson, G.; Biswas, R.; Henseler, K.; Gotz, F.; Zahringer, U.; Peschel, A. A Staphylococcus aureus ypfP mutant with strongly reduced lipoteichoic acid (LTA) content: LTA governs bacterial surface properties and autolysin activity. Mol. Microbiol. 2007, 65, 1078–1091. [Google Scholar] [CrossRef] [Green Version]
- Campbell, J.; Singh, A.K.; Swoboda, J.G.; Gilmore, M.S.; Wilkinson, B.J.; Walker, S. An antibiotic that inhibits a late step in wall teichoic acid biosynthesis induces the cell wall stress stimulon in Staphylococcus aureus. Antimicrob. Agents Chemother. 2012, 56, 1810–1820. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Liu, D.; Singh, A.K.; Drolia, R.; Bai, X.; Tenguria, S.; Bhunia, A.K. Tunicamycin Mediated Inhibition of Wall Teichoic Acid Affects Staphylococcus aureus and Listeria monocytogenes Cell Morphology, Biofilm Formation and Virulence. Front. Microbiol. 2018, 9, 1352. [Google Scholar] [CrossRef]
- Campbell, J.; Singh, A.K.; Santa Maria, J.P., Jr.; Kim, Y.; Brown, S.; Swoboda, J.G.; Mylonakis, E.; Wilkinson, B.J.; Walker, S. Synthetic lethal compound combinations reveal a fundamental connection between wall teichoic acid and peptidoglycan biosyntheses in Staphylococcus aureus. ACS Chem. Biol. 2011, 6, 106–116. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Gill, C.J.; Lee, S.H.; Mann, P.; Zuck, P.; Meredith, T.C.; Murgolo, N.; She, X.; Kales, S.; Liang, L.; et al. Discovery of wall teichoic acid inhibitors as potential anti-MRSA beta-lactam combination agents. Chem. Biol. 2013, 20, 272–284. [Google Scholar] [CrossRef] [Green Version]
- Vickery, C.R.; Wood, B.M.; Morris, H.G.; Losick, R.; Walker, S. Reconstitution of Staphylococcus aureus Lipoteichoic Acid Synthase Activity Identifies Congo Red as a Selective Inhibitor. J. Am. Chem. Soc. 2018, 140, 876–879. [Google Scholar] [CrossRef]
- Richter, S.G.; Elli, D.; Kim, H.K.; Hendrickx, A.P.A.; Sorg, J.A.; Schneewind, O.; Missiakas, D. Small molecule inhibitor of lipoteichoic acid synthesis is an antibiotic for Gram-positive bacteria. Proc. Natl. Acad. Sci. USA 2013, 110, 3531–3536. [Google Scholar] [CrossRef] [Green Version]
- Naclerio, G.A.; Karanja, C.W.; Opoku-Temeng, C.; Sintim, H.O. Antibacterial Small Molecules That Potently Inhibit Staphylococcus aureus Lipoteichoic Acid Biosynthesis. ChemMedChem 2019, 14, 1000–1004. [Google Scholar] [CrossRef]
- Opoku-Temeng, C.; Naclerio, G.A.; Mohammad, H.; Dayal, N.; Abutaleb, N.S.; Seleem, M.N.; Sintim, H.O. N-(1,3,4-oxadiazol-2-yl)benzamide analogs, bacteriostatic agents against methicillin- and vancomycin-resistant bacteria. Eur. J. Med. Chem. 2018, 155, 797–805. [Google Scholar] [CrossRef]
- Opoku-Temeng, C.; Zhou, J.; Zheng, Y.; Su, J.; Sintim, H.O. Cyclic dinucleotide (c-di-GMP, c-di-AMP, and cGAMP) signalings have come of age to be inhibited by small molecules. Chem. Commun. 2016, 52, 9327–9342. [Google Scholar] [CrossRef] [Green Version]
- Rabin, N.; Zheng, Y.; Opoku-Temeng, C.; Du, Y.; Bonsu, E.; Sintim, H.O. Biofilm formation mechanisms and targets for developing antibiofilm agents. Future Med. Chem. 2015, 7, 493–512. [Google Scholar] [CrossRef]
- Sugimoto, S.; Sato, F.; Miyakawa, R.; Chiba, A.; Onodera, S.; Hori, S.; Mizunoe, Y. Broad impact of extracellular DNA on biofilm formation by clinically isolated Methicillin-resistant and -sensitive strains of Staphylococcus aureus. Sci. Rep. 2018, 8, 2254. [Google Scholar] [CrossRef]
- Whitchurch, C.B.; Tolker-Nielsen, T.; Ragas, P.C.; Mattick, J.S. Extracellular DNA required for bacterial biofilm formation. Science 2002, 295, 1487. [Google Scholar] [CrossRef]
- Kavanaugh, J.S.; Flack, C.E.; Lister, J.; Ricker, E.B.; Ibberson, C.B.; Jenul, C.; Moormeier, D.E.; Delmain, E.A.; Bayles, K.W.; Horswill, A.R. Identification of Extracellular DNA-Binding Proteins in the Biofilm Matrix. MBio 2019, 10, e01137-19. [Google Scholar] [CrossRef] [Green Version]
- Foulston, L.; Elsholz, A.K.; DeFrancesco, A.S.; Losick, R. The extracellular matrix of Staphylococcus aureus biofilms comprises cytoplasmic proteins that associate with the cell surface in response to decreasing pH. MBio 2014, 5, e01667-14. [Google Scholar] [CrossRef] [Green Version]
- Elchinger, P.H.; Delattre, C.; Faure, S.; Roy, O.; Badel, S.; Bernardi, T.; Taillefumier, C.; Michaud, P. Effect of proteases against biofilms of Staphylococcus aureus and Staphylococcus epidermidis. Lett. Appl. Microbiol. 2014, 59, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Kaplan, J.B.; LoVetri, K.; Cardona, S.T.; Madhyastha, S.; Sadovskaya, I.; Jabbouri, S.; Izano, E.A. Recombinant human DNase I decreases biofilm and increases antimicrobial susceptibility in staphylococci. J. Antibiot. 2012, 65, 73–77. [Google Scholar] [CrossRef] [Green Version]
- Kiedrowski, M.R.; Kavanaugh, J.S.; Malone, C.L.; Mootz, J.M.; Voyich, J.M.; Smeltzer, M.S.; Bayles, K.W.; Horswill, A.R. Nuclease modulates biofilm formation in community-associated methicillin-resistant Staphylococcus aureus. PLoS ONE 2011, 6, e26714. [Google Scholar] [CrossRef] [Green Version]
- Darouiche, R.O.; Mansouri, M.D.; Gawande, P.V.; Madhyastha, S. Antimicrobial and antibiofilm efficacy of triclosan and DispersinB combination. J. Antimicrob. Chemother. 2009, 64, 88–93. [Google Scholar] [CrossRef]
- Kaplan, J.B.; Ragunath, C.; Ramasubbu, N.; Fine, D.H. Detachment of Actinobacillus actinomycetemcomitans biofilm cells by an endogenous beta-hexosaminidase activity. J. Bacteriol. 2003, 185, 4693–4698. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, T.; Swoboda, J.G.; Campbell, J.; Walker, S.; Gilmore, M.S. In vitro antimicrobial activity of wall teichoic acid biosynthesis inhibitors against Staphylococcus aureus isolates. Antimicrob. Agents Chemother. 2011, 55, 767–774. [Google Scholar] [CrossRef] [Green Version]
- Nair, S.; Desai, S.; Poonacha, N.; Vipra, A.; Sharma, U. Antibiofilm Activity and Synergistic Inhibition of Staphylococcus aureus Biofilms by Bactericidal Protein P128 in Combination with Antibiotics. Antimicrob. Agents Chemother. 2016, 60, 7280–7289. [Google Scholar]
- Eldesouky, H.E.; Li, X.Y.; Abutaleb, N.S.; Mohammad, H.; Seleem, M.N. Synergistic interactions of sulfamethoxazole and azole antifungal drugs against emerging multidrug-resistant Candida auris. Int. J. Antimicrob. Agents 2018, 52, 754–761. [Google Scholar] [CrossRef]
- Mohammad, H.; Cushman, M.; Seleem, M.N. Antibacterial Evaluation of Synthetic Thiazole Compounds In Vitro and In Vivo in a Methicillin-Resistant Staphylococcus aureus (MRSA) Skin Infection Mouse Model. PLoS ONE 2015, 10, e0142321. [Google Scholar] [CrossRef] [Green Version]
- Meletiadis, J.; Pournaras, S.; Roilides, E.; Walsh, T.J. Defining Fractional Inhibitory Concentration Index Cutoffs for Additive Interactions Based on Self-Drug Additive Combinations, Monte Carlo Simulation Analysis, and In Vitro-In Vivo Correlation Data for Antifungal Drug Combinations against Aspergillus fumigatus. Antimicrob. Agents Chemother. 2010, 54, 602–609. [Google Scholar]
Sample Availability: Samples of HSGN-94 and HSGN-189 are available from the authors as stocks last. |




| A | MRSA ATCC 33592 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC Alone | Combination MIC | ∑FICI | SYN/ADD/IND | MIC Alone | Combination MIC | ∑FICI | SYN/ADD/IND | |||||
| Antibiotic | Antibiotic | HSGN-94 | Antibiotic | HSGN-94 | Antibiotic | HSGN-189 | Antibiotic | HSGN-189 | ||||
| Targocil | 16 | 0.5 | 2 | 0.25 | 0.6 | ADD | 32 | 0.5 | 16 | 0.5 | 1.5 | IND |
| Tunicamycin | 256 | 0.5 | 64 | 0.125 | 0.5 | SYN | 256 | 0.5 | 32 | 0.25 | 0.6 | ADD |
| B | MRSA USA300 | |||||||||||
| MIC Alone | Combination MIC | ∑FICI | SYN/ADD/IND | MIC Alone | Combination MIC | ∑FICI | SYN/ADD/IND | |||||
| Antibiotic | Antibiotic | HSGN-94 | Antibiotic | HSGN-94 | Antibiotic | HSGN-189 | Antibiotic | HSGN-189 | ||||
| Targocil | >1024 | 2 | 16 | 2 | 1.0 | ADD | >1024 | 2 | 16 | 2 | 1.0 | ADD |
| Tunicamycin | 32 | 2 | 2 | 1 | 0.6 | ADD | 64 | 2 | 4 | 0.5 | 0.3 | SYN |
| C | VRE Faecalis ATCC 51575 | |||||||||||
| MIC Alone | Combination MIC | ∑FICI | SYN/ADD/IND | MIC Alone | Combination MIC | ∑FICI | SYN/ADD/IND | |||||
| Antibiotic | Antibiotic | HSGN-94 | Antibiotic | HSGN-94 | Antibiotic | HSGN-189 | Antibiotic | HSGN-189 | ||||
| Targocil | >1024 | 2 | 16 | 2 | 1.0 | ADD | >1024 | 2 | 16 | 2 | 1.0 | IND |
| Tunicamycin | 16 | 2 | 4 | 0.5 | 0.5 | SYN | 16 | 2 | 0.5 | 1 | 0.5 | SYN |
| A | MRSA USA300 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MBIC Alone | Combination MBIC | ∑FICI | SYN/ADD/IND | MBIC Alone | Combination MBIC | ∑FICI | SYN/ADD/IND | |||||
| Antibiotic | Antibiotic | HSGN-94 | Antibiotic | HSGN-94 | Antibiotic | HSGN-189 | Antibiotic | HSGN-189 | ||||
| Tunicamycin | 64 | 2 | 2 | 1 | 0.5 | SYN | 64 | 2 | 4 | 0.5 | 0.3 | SYN |
| B | VRE Faecalis ATCC 51575 | |||||||||||
| MBIC Alone | Combination MBIC | ∑FICI | SYN/ADD/IND | MBIC Alone | Combination MBIC | ∑FICI | SYN/ADD/IND | |||||
| Antibiotic | Antibiotic | HSGN-94 | Antibiotic | HSGN-94 | Antibiotic | HSGN-189 | Antibiotic | HSGN-189 | ||||
| Tunicamycin | 32 | 2 | 8 | 0.06 | 0.3 | SYN | 32 | 2 | 8 | 0.06 | 0.3 | SYN |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naclerio, G.A.; Onyedibe, K.I.; Sintim, H.O. Lipoteichoic Acid Biosynthesis Inhibitors as Potent Inhibitors of S. aureus and E. faecalis Growth and Biofilm Formation. Molecules 2020, 25, 2277. https://doi.org/10.3390/molecules25102277
Naclerio GA, Onyedibe KI, Sintim HO. Lipoteichoic Acid Biosynthesis Inhibitors as Potent Inhibitors of S. aureus and E. faecalis Growth and Biofilm Formation. Molecules. 2020; 25(10):2277. https://doi.org/10.3390/molecules25102277
Chicago/Turabian StyleNaclerio, George A., Kenneth I. Onyedibe, and Herman O. Sintim. 2020. "Lipoteichoic Acid Biosynthesis Inhibitors as Potent Inhibitors of S. aureus and E. faecalis Growth and Biofilm Formation" Molecules 25, no. 10: 2277. https://doi.org/10.3390/molecules25102277
APA StyleNaclerio, G. A., Onyedibe, K. I., & Sintim, H. O. (2020). Lipoteichoic Acid Biosynthesis Inhibitors as Potent Inhibitors of S. aureus and E. faecalis Growth and Biofilm Formation. Molecules, 25(10), 2277. https://doi.org/10.3390/molecules25102277