The Use of Macro, Micro, and Trace Elemental Profiles to Differentiate Commercial Single Vineyard Pinot noir Wines at a Sub-Regional Level
Abstract
:1. Introduction
2. Results and Discussion
2.1. Method Validation and Overall Results
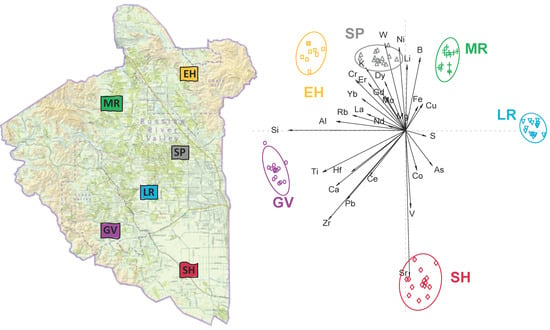
2.2. Wine Elemental Fingerprints Differ Significantly by Neighborhood and Over Two Separate Vintages
2.3. A New Neighborhood Was Classified as Falling Outside the Existing Neighborhoods Based on Its Elemental Fingerprints
3. Materials and Methods
3.1. Materials and Reagents
3.2. Macroelemental Profiling with Microwave Plasma-Atomic Emission Spectroscopy (MP-AES)
3.3. Elemental Profiling with Inductively Coupled Plasma-Mass Spectrometry (ICP-MS)
3.4. Data Treatment and Statistical Methods
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Douro Valley History of Douro Wines. Available online: http://www.dourovalley.eu/en/PageGen.aspx?WMCM_PaginaId=79479 (accessed on 12 July 2019).
- American Viticultural Areas. Code of Federal Regulations; Part 9, Title 27, Subpart C. 2019. Available online: https://ecfr.io/Title-27/sp27.1.9.c (accessed on 12 July 2019).
- Regulations and Rulings Division. Established American Viticultural Areas. 2019; Alcohol and Tabaco Tax and Trade Bureau. Available online: https://www.ttb.gov/wine/established-avas (accessed on 15 May 2020).
- Atkin, T.; Johnson, R. Appellation as an indicator of quality. Int. J. Wine Bus. Res. 2010, 22, 42–61. [Google Scholar] [CrossRef] [Green Version]
- Johnson, R.; Bruwer, J. Regional brand image and perceived wine quality: The consumer perspective. Int. J. Wine Bus. Res. 2007, 19, 276–297. [Google Scholar] [CrossRef]
- Giaccio, M.; Vicentini, A. Determination of the geographical origin of wines by means of the mineral content and the stable isotope ratios: A review. J. Commodity Sci. Technol. Qual. 2008, 47, 267–284. [Google Scholar]
- Ellis, D.I.; Brewster, V.L.; Dunn, W.B.; Allwood, J.W.; Golovanov, A.P.; Goodacre, R. Fingerprinting food: Current technologies for the detection of food adulteration and contamination. Chem. Soc. Rev. 2012, 41, 5706. [Google Scholar] [CrossRef] [PubMed]
- Kelly, S.; Heaton, K.; Hoogewerff, J. Tracing the Geographical Origin of Food: The Application of Multi-Element and Multi-Isotope Analysis. Trends Food Sci. Technol. 2005, 16, 555–567. [Google Scholar] [CrossRef]
- Raco, B.; Dotsika, E.; Poutoukis, D.; Battaglini, R.; Chantzi, P. O–H–C Isotope Ratio Determination in Wine in Order to Be Used as a Fingerprint of Its Regional Origin. Food Chem. 2015, 168, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Christoph, N.; Rossmann, A.; Voerkelius, S. Possibilities and Limitations of Wine Authentication Using Stable Isotope and Meteorological Data, Data Banks and Statistical Tests. Part 1: Wines from Franconia and Lake Constance 1992 to 2001. Mitteilungen Klosterneubg. 2003, 53, 23–40. [Google Scholar]
- Christoph, N.; Rossmann, A.; Schlicht, C.; Voerkelius, S. Wine Authentication Using Stable Isotope Ratio Analysis: Significance of Geographic Origin, Climate, and Viticultural Parameters. ACS Symp. Ser. 2007, 952, 166–179. [Google Scholar]
- Pohl, P. What do metals tell us about wine? TrAC Trends Anal. Chem. 2007, 26, 941–949. [Google Scholar] [CrossRef]
- Jos, A.; Moreno, I.; Gonzalez, A.G.; Repetto, G.; Camean, A.M. Differentiation of sparkling wines (cava and champagne) according to their mineral content. Talanta 2004, 63, 377–382. [Google Scholar] [CrossRef]
- Thiel, G.; Geisler, G.; Blechschmidt, I.; Danzer, K. Determination of trace elements in wines and classification according to their provenance. Anal. Bioanal. Chem. 2004, 378, 1630–1636. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.E.; Watling, R.J.; Lee, G.S. The multi-element determination and regional discrimination of Australian wines. Food Chem. 2012, 133, 1081–1089. [Google Scholar] [CrossRef]
- Sperkova, J.; Suchanek, M. Multivariate classification of wines from different Bohemian regions (Czech Republic). Food Chem. 2005, 93, 659–663. [Google Scholar] [CrossRef]
- Rocha, S.; Pinto, E.; Almeida, A.; Fernandes, E. Multi-elemental analysis as a tool for characterization and differentiation of Portuguese wines according to their Protected Geographical Indication. Food Control 2019, 103, 27–35. [Google Scholar] [CrossRef]
- Mirabal-Gallardo, Y.; Caroca-Herrera, M.A.; Muñoz, L.; Meneses, M.; Laurie, V.F. Multi-element analysis and differentiation of Chilean wines using mineral composition and multivariate statistics. Cienc. e Investig. Agrar. 2018, 45, 181–191. [Google Scholar] [CrossRef] [Green Version]
- Šelih, V.S.; Šala, M.; Drgan, V. Multi-element analysis of wines by ICP-MS and ICP-OES and their classification according to geographical origin in Slovenia. Food Chem. 2014, 153, 414–423. [Google Scholar] [CrossRef]
- Galgano, F.; Favati, F.; Caruso, M.; Scarpa, T.; Palma, A. Analysis of trace elements in southern Italian wines and their classification according to provenance. LWT Food Sci. Technol. 2008, 41, 1808–1815. [Google Scholar] [CrossRef]
- Serapinas, P.; Venskutonis, P.R.; Aninkevičius, V.; Ežerinskis, Ž.; Galdikas, A.; Juzikienė, V. Step by step approach to multi-element data analysis in testing the provenance of wines. Food Chem. 2008, 107, 1652–1660. [Google Scholar] [CrossRef]
- Pérez-Álvarez, E.P.; Garcia, R.; Barrulas, P.; Dias, C.; Cabrita, M.J.; Garde-Cerdán, T. Classification of wines according to several factors by ICP-MS multi-element analysis. Food Chem. 2019, 270, 273–280. [Google Scholar] [CrossRef]
- Coetzee, P.P.; Van Jaarsveld, F.P.; Vanhaecke, F. Intraregional classification of wine via ICP-MS elemental fingerprinting. Food Chem. 2014, 164, 485–492. [Google Scholar] [CrossRef]
- Baxter, M.J.; Crews, H.M.; Dennis, M.J.; Goodall, I.; Anderson, D. The determination of the authenticity of wine from its trace element composition. Food Chem. 1997, 60, 443–450. [Google Scholar] [CrossRef]
- Geana, I.; Iordache, A.; Ionete, R.; Marinescu, A.; Ranca, A.; Culea, M. Geographical origin identification of Romanian wines by ICP-MS elemental analysis. Food Chem. 2013, 138, 1125–1134. [Google Scholar] [CrossRef] [PubMed]
- Hopfer, H.; Nelson, J.; Collins, T.S.; Heymann, H.; Ebeler, S.E. The combined impact of vineyard origin and processing winery on the elemental profile of red wines. Food Chem. 2015, 172, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Greenough, J.D.; Longerich, H.P.; Jackson, S.E. Element fingerprinting of Okanagan Valley wines using ICP – MS: Relationships between wine composition, vineyard and wine colour. Aust. J. Grape Wine Res. 1997, 3, 75–83. [Google Scholar] [CrossRef]
- Redan, B.W.; Jablonski, J.E.; Halverson, C.; Jaganathan, J.; Mabud, M.A.; Jackson, L.S. Factors Affecting Transfer of the Heavy Metals Arsenic, Lead, and Cadmium from Diatomaceous-Earth Filter Aids to Alcoholic Beverages during Laboratory-Scale Filtration. J. Agric. Food Chem. 2019, 67, 2670–2678. [Google Scholar] [CrossRef]
- Dinca, O.R.; Ionete, R.E.; Costinel, D.; Geana, I.E.; Popescu, R.; Stefanescu, I.; Radu, G.L. Regional and Vintage Discrimination of Romanian Wines Based on Elemental and Isotopic Fingerprinting. Food Anal. Methods 2016, 9, 2406–2417. [Google Scholar] [CrossRef]
- Kment, P.; Mihaljevič, M.; Ettler, V.; Šebek, O.; Strnad, L.; Rohlová, L. Differentiation of Czech wines using multielement composition - A comparison with vineyard soil. Food Chem. 2005, 91, 157–165. [Google Scholar] [CrossRef]
- Nelson, J.; Hopfer, H.; Gilleland, G.; Cuthbertson, D.; Boulton, R.; Ebeler, S.E. Elemental Profiling of Malbec Wines under Controlled Conditions Using Microwave Plasma-Atomic Emission Spectroscopy. Am. J. Enol. Vitic. 2015, 66, 373–378. [Google Scholar] [CrossRef]
- Taylor, V.F.; Longerich, H.P.; Greenough, J.D. Multielement analysis of Canadian wines by inductively coupled plasma mass spectrometry (ICP-MS) and multivariate statistics. J. Agric. Food Chem. 2003, 51, 856–860. [Google Scholar] [CrossRef]
- Marengo, E.; Aceto, M. Statistical investigation of the differences in the distribution of metals in Nebbiolo-based wines. Food Chem. 2003, 81, 621–630. [Google Scholar] [CrossRef]
- Castiñeira Gómez, M.D.M.; Feldmann, I.; Jakubowski, N.; Andersson, J.T. Classification of German White Wines with Certified Brand of Origin by Multielement Quantitation and Pattern Recognition Techniques. J. Agric. Food Chem. 2004, 52, 2962–2974. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, S.M.; Otero, M.; Alves, A.A.; Coimbra, J.; Coimbra, M.A.; Pereira, E.; Duarte, A.C. Elemental analysis for categorization of wines and authentication of their certified brand of origin. J. Food Compos. Anal. 2011, 24, 548–562. [Google Scholar] [CrossRef]
- Angus, N.S.; Keeffe, T.J.O.; Stuart, K.R.; Miskelly, G.M.; Keefe, O. Regional classification of New Zealand red wines using inductively-coupled plasma-mass spectrometry (ICP-MS). Aust. J. Grape Wine Res. 2006, 12, 170–176. [Google Scholar] [CrossRef]
- Coetzee, P.P.; Steffens, F.E.; Eiselen, R.J.; Augustyn, O.P.; Balcaen, L.; Vanhaecke, F. Multi-element analysis of South African wines by ICP-MS and their classification according to geographical origin. J. Agric. Food Chem. 2005, 53, 5060–5066. [Google Scholar] [CrossRef]
- Coetzee, P.P.; Vanhaecke, F. Classifying wine according to geographical origin via quadrupole-based ICP-mass spectrometry measurements of boron isotope ratios. Anal. Bioanal. Chem. 2005, 383, 977–984. [Google Scholar] [CrossRef]
- Perez, A.L.; Smith, B.W.; Anderson, K.A. Stable isotope and trace element profiling combined with classification models to differentiate geographic growing origin for three fruits: Effects of subregion and variety. J. Agric. Food Chem. 2006, 54, 4506–4516. [Google Scholar] [CrossRef]
- Fan, S.; Zhong, Q.; Gao, H.; Wang, D.; Li, G.; Huang, Z. Elemental profile and oxygen isotope ratio (δ 18 O) for verifying the geographical origin of Chinese wines. J. Food Drug Anal. 2018, 26, 1033–1044. [Google Scholar] [CrossRef] [Green Version]
- Dutra, S.V.; Adami, L.; Marcon, A.R.; Carnieli, G.J.; Roani, C.A.; Spinelli, F.R.; Leonardelli, S.; Ducatti, C.; Moreira, M.Z.; Vanderlinde, R. Determination of the geographical origin of Brazilian wines by isotope and mineral analysis. Anal. Bioanal. Chem. 2011, 401, 1571–1576. [Google Scholar] [CrossRef]
- Orellana, S.; Johansen, A.M.; Gazis, C. Geographic classification of U.S. Washington State wines using elemental and water isotope composition. Food Chem. X 2019, 1, 100007. [Google Scholar] [CrossRef]
- Boone, V. A Guide to California’s Russian River Valley. Available online: https://www.winemag.com/2015/06/04/making-sense-of-the-russian-river-valley/ (accessed on 2 March 2020).
- Volpe, M.G.; La Cara, F.; Volpe, F.; De Mattia, A.; Serino, V.; Petitto, F.; Zavalloni, C.; Limone, F.; Pellecchia, R.; De Prisco, P.P.; et al. Heavy Metal Uptake in the Enological Food Chain. Food Chem. 2009, 117, 553–560. [Google Scholar] [CrossRef]
- Aceto, M.; Bonello, F.; Musso, D.; Tsolakis, C.; Cassino, C.; Osella, D. Wine Traceability with Rare Earth Elements. Beverages 2018, 4, 23. [Google Scholar] [CrossRef] [Green Version]
- Jakubowski, N.; Brandt, R.; Stuewer, D.; Eschnauer, H.R.; Gortges, S. Analysis of Wines by ICP-MS: Is the Pattern of the Rare Earth Elements a Reliable Fingerprint for the Provenance? Fresenius. J. Anal. Chem. 1999, 364, 424–428. [Google Scholar] [CrossRef]
- Thomsen, V.; Schatzlein, D.; Mercuro, D. Limits of Detection in Spectroscopy. Pure Appl. Chem. 2003, 18, 112–114. [Google Scholar]
- Fox, J.; Weissberg, S. An {R} Companion to Applied Regression, 2nd ed.; Sage: Thousand Oaks, CA, USA, 2011. [Google Scholar]
- Lenth, R. emmeans: Estimated Marginal Means, aka Least-Squares Means. Available online: https://cran.r-project.org/package=emmeans (accessed on 10 May 2019).
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friendly, M.; Fox, J. candisc: Visualizing Generalized Canonical Discriminant and Canonical Correlation Analysis. Available online: https://cran.r-project.org/package=candisc (accessed on 5 May 2019).
- Kucheryavskiy, S. mdatools: Multivariate Data Analysis for Chemometrics. Available online: https://cran.r-project.org/package=mdatools (accessed on 5 May 2019).
Sample Availability: Samples of the compounds are not available from the authors. |


| GV (μg/kg) | LR (μg/kg) | MR (μg/kg) | SH (μg/kg) | SP (μg/kg) | |
|---|---|---|---|---|---|
| Li | 5.39 ab [2.91,8.58] | 4.60 a [< 2.02,7.8] | 2.83 a [N.D.,6.03] | 3.65 a [N.D.,6.84] | 3.65 a [6.47,12.9] |
| V | 0.35 ab [0.18,0.51] | 0.18 a [N.D.,0.34] | 0.53 b [0.36,0.70] | 0.29 ab [N.D.,0.46] | 0.29 ab [0.36,0.70] |
| Co | 6.51 b [4.53,8.49] | 10.7 c [8.76,12.7] | 2.33 a [0.35,4.31] | 6.40 b [4.42,8.38] | 6.40 b [2.12,6.08] |
| Ni | 31.4 ab [21.0,41.7] | 62.7 d [52.3,73.0] | 24.4 a [14.0,34.8] | 42.1 bc [31.7,52.5] | 42.1 bc [44.9,65.7] |
| Ga | 0.09 b [0.06,0.13] | 0.06 ab [0.03,0.09] | 0.09 ab [0.05,0.12] | 0.04 a [< 0.02,0.08] | 0.04 a [0.06,0.12] |
| Mo | 1.75 b [0.83,2.66] | 0.81 ab [N.D.,1.73] | 0.36 a [N.D.,1.27] | 0.56 ab [N.D.,1.48] | 0.56 ab [N.D.,1.32] |
| Cd | 0.22 d [0.19,0.26] | 0.15 bc [0.12,0.19] | 0.07 a [0.03,0.11] | 0.16 c [0.12,0.20] | 0.16 c [0.06,0.13] |
| Sb | 0.09 b [0.06,0.13] | 0.05 ab [< 0.03,0.08] | < 0.03 a [N.D.,0.05] | 0.05 ab [< 0.03,0.08] | 0.05 ab [N.D.,0.06] |
| Cs | 5.58 a [1.88,9.29] | 3.41 a [N.D.,7.11] | 4.28 a [0.57,7.98] | 2.43 a [N.D.,6.14] | 2.43 a [11.1,18.5] |
| Ba | 324 abc [231,418] | 422 bc [328,515] | 234 a [141,327] | 298 ab [205,391] | 298 ab [363,549] |
| Ce | 0.08 b [0.05,0.10] | 0.08 b [0.05,0.10] | 0.03 a [N.D.,0.05] | 0.03 a [0.01,0.06] | 0.03 a [0.02,0.07] |
| Nd | 0.07 c [0.05,.0.09] | 0.06 bc [0.04,0.08] | 0.02 a [N.D.,0.04] | 0.03 a [N.D.,0.05] | 0.03 a [0.01,0.06] |
| Ta | 0.27 b [0.17,0.37] | 0.01 a [N.D.,0.10] | N.D. a [N.D.,0.10] | 0.05 ab [N.D.,0.15] | 0.05 ab [N.D.,0.14] |
| W | 0.31 b [0.18,0.43] | 0.09 a [N.D.,0.22] | 0.15 ab [0.02,0.28] | 0.13 ab [N.D.,0.26] | 0.13 ab [0.16,0.41] |
| Tl | 0.52 b [0.32,0.72] | 0.35 ab [0.15,0.55] | 0.14 a [N.D.,0.34] | 0.38 ab [0.18,0.58] | 0.38 ab [0.20,0.60] |
| Pb 1 | 6.25 c [4.28,8.23] | 3.06 ab [1.09,5.04] | 1.40 a [N.D.,3.38] | 5.22 bc [3.24,7.19] | 5.22 bc [0.99,4.94] |
| GV (mg/kg) | LR (mg/kg) | MR (mg/kg) | SH (mg/kg) | SP (mg/kg) | |
| P | 300 a [263,337] | 298 a [261,335] | 270 a [233,307] | 304 ab [267,341] | 356 b [319,393] |
| B | 4.96 a [3.49,6.44] | 5.80 ab [4.32,7.27] | 8.44 c [6.97,9.91] | 4.01 a [2.53,5.48] | 7.70 bc [6.22,9.17] |
| Si | 21.4 a [17.8,25.1] | 18.1 a [14.5,21.7] | 16.8 a [13.2,20.5] | 19.2 a [15.6,22.8] | 27.3 b [23.6,30.9] |
| Ca | 60.3 b [54.8,65.8] | 45.8 a [40.3,51.2] | 50.20 a [44.7,55.6] | 49.6 a [44.1,55.1] | 49.1 a [43.6,54.5] |
| Mn | 3.15 b [2.50,3.79] | 2.65 b [2.00,3.29] | 1.51 a [0.87,2.16] | 2.71 b [2.06,3.36] | 2.45 ab [1.80,3.10] |
| Sr | 0.99 ab [0.73,1.26] | 1.08 b [0.82,1.34] | 0.83 ab [0.57,1.09] | 1.58 c [1.32,1.84] | 0.64 a [0.38,0.9] |
| K | 467 a [410,525] | 490 ab [432,548] | 557 bc [499,614] | 526 ab [468,583] | 620 c [563,678] |
| Rb | 1.42 ab [0.89,1.96] | 1.62 b [1.08,2.15] | 0.75 a [0.22,1.29] | 1.36 ab [0.83,1.89] | 2.48 c [1.95,3.02] |
| GV (μg/kg) | LR (μg/kg) | MR (μg/kg) | SH (μg/kg) | SP (μg/kg) | EH (μg/kg) | |
|---|---|---|---|---|---|---|
| Li | 6.02 ab [< 0.47,11.9] | 5.49 ab [N.D.,11.4] | 12.1 b [6.22,18.0] | 1.95 a [N.D.,7.82] | 8.03 ab [2.16,13.9] | 6.35 ab [N.D.,13.9] |
| B | 6286 ab [4994,7578] | 6604 ab [5312,7896] | 7507 b [6215,8800] | 5255 a [3963,6548] | 7651 b [6359,8943] | 5538 ab [3870,7207] |
| Al | 204 bc [168,240] | 131 a [94.6,167] | 226 bc [190,262] | 222 bc [186,258] | 201 b [164,237] | 264 c [218,311] |
| Ti | 17.4 b [16.0,18.8] | 12.6 a [11.2,14.0] | 12.7 a [11.3,14.1] | 13.2 a [11.8,14.6] | 12.0 a [10.6,13.5] | 14.4 a [12.6,16.2] |
| V | < 0.01 a [N.D.,0.14] | < 0.01 a [N.D.,0.14] | < 0.01 a [N.D.,0.14] | 0.22 b [0.09,0.36] | < 0.01 a [N.D.,0.14] | < 0.01 ab [N.D.,0.18] |
| Cr | 4.24 bc [2.70,5.78] | 1.55 a [< 0.20,3.09] | 5.96 c [4.42,7.50] | 3.24 ab [1.70,4.78] | 3.80 abc [2.26,5.34] | 5.05 bc [3.06,7.04] |
| Mn | 1892 ab [1343,2442] | 1740 ab [1190,2289] | 1474 a [925,2024] | 2109 ab [1559,2658] | 2002 ab [1453,2552] | 2451 b [1741,3160] |
| Fe | 684 a [411,956] | 723 a [450,995] | 1636 b [1324,1909] | 1244 bc [972,1517] | 1151 b [879,1424] | 9412 ab [590,1294] |
| Co | 4.19 ab [2.37,6.00] | 5.50 b [3.69,7.31] | 2.20 a [0.38,4.01] | 4.52 ab [2.70,6.33] | 2.79 ab [0.98,4.60] | 5.36 ab [3.02,7.70] |
| Ni | 24.8 a [16.9,32.8] | 34.7 ab [26.8,42.6] | 32.1 a [24.2,40.0] | 30.8 a [22.9,38.7] | 45.4 bc [37.4,53.3] | 54.3 c [44.1,64.5] |
| Cu | 29.4 a [1.36,57.4] | 44.5 a [16.5,72.5] | 45.3 a [17.2,73.3] | 52.5 ab [24.5,80.5] | 92.8 b [64.8,121] | 29.0 a [N.D.,65.2] |
| As | 1.36 b [0.53,2.20] | 2.25 b [1.41,3.08] | < 0.17 b [N.D.,0.84] | 1.13 ab [0.30,1.96] | 1.07 ab [0.24,1.91] | 0.91 ab [N.D.,1.99] |
| Rb | 1842 ab [1542,2232] | 1468 a [1078,1857] | 1404 a [1015,1794] | 1809 ab [1420,2199] | 2346 b [1956,2736] | 2075 ab [1572,2578] |
| Sr | 852 a [609,1094] | 750 a [507,992] | 702 a [460,945] | 1558 b [1316,1801] | 573 a [331,816] | 675 a [362,988] |
| Zr | 2.96 b [2.44,3.49] | 1.43 a [0.91,1.96] | 1.87 a [1.34,2.40] | 2.91 b [2.39,3.44] | 1.43 a [0.91,1.96] | 2.88 b [2.20,3.56] |
| Mo | 4.75 ab [0.66,8.85] | 0.22 a [N.D.,4.31] | 6.56 b [2.47,10.7] | 0.29 a [N.D.,4.38] | 2.68 ab [N.D.,6.77] | N.D. ab [N.D.,5.28] |
| La | 0.06 b [0.03,0.09] | 0.05 ab [0.02,0.07] | 0.02 a [N.D.,0.04] | 0.03 ab [0.01,0.06] | 0.06 ab [0.03,0.08] | 0.07 b [0.03,0.10] |
| Ce | 0.11 b [0.06,0.15] | 0.08 ab [0.03,0.12] | 0.03 a [N.D.,0.08] | 0.11 b [0.06,0.16] | 0.09 ab [0.04,0.13] | 0.12 b [0.06,0.18] |
| Nd | 0.05 b [0.03,0.08] | 0.05 b [0.03,0.07] | 0.01 a [N.D.,0.03] | 0.02 ab [N.D.,0.04] | 0.04 ab [0.02,0.06] | 0.05 ab [0.02,0.07] |
| Gd | 0.02 abc [N.D.,0.03] | 0.02 bc [N.D.,0.03] | <0.02 a [N.D.,<0.02] | <0.02 ab [N.D.,0.02] | 0.02 abc [N.D.,0.02] | 0.03 c [N.D.,0.04] |
| Dy | 0.02 a [0.01,0.04] | 0.03 ab [0.01,0.04] | 0.01 a [N.D.,0.03] | 0.01 ab [N.D.,0.03] | 0.03 a [0.01,0.04] | 0.05 b [0.03,0.07] |
| Er | 0.03 bc [0.02,0.04] | 0.03 abc [0.01,0.04] | 0.01 ab [N.D., 0.02] | 0.01 ab [N.D.,0.02] | 0.03 abc [0.01,0.04] | 0.05 c [0.03,0.06] |
| Yb | 0.04 b [0.03,0.05] | 0.03 ab [0.02,0.04] | <0.01 a [<0.01,0.03] | 0.02 a [<0.01,0.03] | 0.03 ab [0.02,0.04] | 0.04 b [0.03,0.06] |
| Hf | 0.13 b 0.09,0.17] | 0.07 a [0.03,0.11] | 0.10 ab [0.06,0.14] | 0.13 ab [0.09,0.17] | 0.08 ab [0.04,0.12] | 0.14 ab [0.09,0.19] |
| W | 0.03 ab [0.03,0.04] | 0.03 a [0.02,0.04] | 0.05 b [0.04,0.05] | 0.03 ab [0.02,0.04] | 0.04 ab [0.03,0.05] | 0.04 ab [0.03,0.05] |
| Pb 1 | 3.40 c [2.15,4.65] | 1.44 ab [0.19,2.69] | 0.46 a [N.D.,1.71] | 3.08 bc [1.83,4.33] | 1.86 abc [0.61,3.11] | 2.99 bc [1.38,4.60] |
| GV (mg/kg) | LR (mg/kg) | MR (mg/kg) | SH (mg/kg) | SP (mg/kg) | EH (mg/kg) | |
| Mg | 123 a [108,138] | 132 ab [117,147] | 127 a [112,142] | 133 ab [118,148] | 124 a [109,139] | 156 b [137,175] |
| K | 913 ab [788,1037] | 790 a [665,914] | 993 b [868,1117] | 855 ab [730,979] | 1022 b [898,1147] | 984 ab [823,1145] |
| Ca | 73.8 b [66.6,81.0] | 53.5 a [48.2,62.6] | 55.4 a [48.2,62.6] | 58.9 a [51.7,66.1] | 50.0 a [42.8,57.2] | 59.6 a [50.3,68.9] |
| Si | 28.5 a [25.8,31.1] | 18.6 a [16.0,21.3] | 21.7 a [19.0,24.3] | 25.8 b [23.1,28.4] | 26.3 b [23.6,28.9] | 34.1 c [30.7,37.5] |
| S | 118 ab [98.8,136] | 112 ab [93.5,131] | 130 b [111,149] | 107 ab [88.0,126] | 99.1 a [80.3,118] | 85.0 a [60.7,109] |
| Classes | |||||
|---|---|---|---|---|---|
| GV | LR | MR | SH | SP | |
| Model components | 3 | 3 | 3 | 3 | 3 |
| Cumulative variance (%) | 99.97 | 99.99 | 99.99 | 99.97 | 99.99 |
| Specificity (%) | 96.7 | 96.8 | 73.3 | 90.3 | 96.7 |
| Sensitivity (%) | 100 | 100 | 100 | 100 | 100 |
| Accuracy (%) | 100 | 97.4 | 94.7 | 100 | 94.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanabe, C.K.; Nelson, J.; Boulton, R.B.; Ebeler, S.E.; Hopfer, H. The Use of Macro, Micro, and Trace Elemental Profiles to Differentiate Commercial Single Vineyard Pinot noir Wines at a Sub-Regional Level. Molecules 2020, 25, 2552. https://doi.org/10.3390/molecules25112552
Tanabe CK, Nelson J, Boulton RB, Ebeler SE, Hopfer H. The Use of Macro, Micro, and Trace Elemental Profiles to Differentiate Commercial Single Vineyard Pinot noir Wines at a Sub-Regional Level. Molecules. 2020; 25(11):2552. https://doi.org/10.3390/molecules25112552
Chicago/Turabian StyleTanabe, Courtney K., Jenny Nelson, Roger B. Boulton, Susan E. Ebeler, and Helene Hopfer. 2020. "The Use of Macro, Micro, and Trace Elemental Profiles to Differentiate Commercial Single Vineyard Pinot noir Wines at a Sub-Regional Level" Molecules 25, no. 11: 2552. https://doi.org/10.3390/molecules25112552
APA StyleTanabe, C. K., Nelson, J., Boulton, R. B., Ebeler, S. E., & Hopfer, H. (2020). The Use of Macro, Micro, and Trace Elemental Profiles to Differentiate Commercial Single Vineyard Pinot noir Wines at a Sub-Regional Level. Molecules, 25(11), 2552. https://doi.org/10.3390/molecules25112552