In Vitro Metabolic Transformation of Pharmaceuticals by Hepatic S9 Fractions from Common Carp (Cyprinus carpio)
Abstract
:1. Introduction
2. Results
2.1. Characterization of Piscine and Ovine S9 Fraction
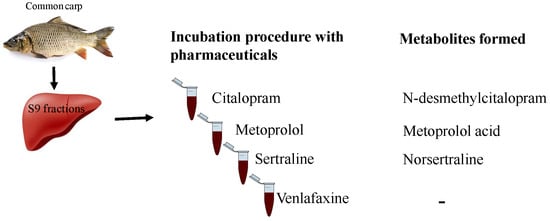
2.2. Metabolites Formation
2.3. N-desmethylcitalopram Formation
2.4. Inhibition Study
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Animals
4.3. Preparation of S9 Fraction
4.4. S9 Fraction Characterization
4.5. In Vitro Incubation of S9 Fraction with Pharmaceutical Compounds
4.6. The Kinetic Study Focused on Citalopram
4.7. Inhibition Study Focused on Citalopram
4.8. Liquid Chromatography-High Resolution Mass Spectrometry (LC-HRMS)
4.9. Data Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stroski, K.M.; Luong, K.H.; Challis, J.K.; Chaves-Barquero, L.G.; Hanson, M.L.; Wong, C.S. Wastewater sources of per- and polyfluorinated alkyl substances (PFAS) and pharmaceuticals in four Canadian Arctic communities. Sci. Total Environ. 2020, 708, 134494. [Google Scholar] [CrossRef] [PubMed]
- Tröger, R.; Köhler, S.J.; Franke, V.; Bergstedt, O.; Wiberg, K. A case study of organic micropollutants in a major Swedish water source – Removal efficiency in seven drinking water treatment plants and influence of operational age of granulated active carbon filters. Sci. Total Environ. 2020, 706, 135680. [Google Scholar] [CrossRef] [PubMed]
- Batt, A.L.; Kincaid, T.M.; Kostich, M.S.; Lazorchak, J.M.; Olsen, A.R. Evaluating the extent of pharmaceuticals in surface waters of the United States using a National-scale Rivers and Streams Assessment survey. Environ. Toxicol. Chem. 2016, 35, 874–881. [Google Scholar] [CrossRef] [PubMed]
- Petrie, B.; Barden, R.; Kasprzyk-Hordern, B. A review on emerging contaminants in wastewaters and the environment: Current knowledge, understudied areas and recommendations for future monitoring. Water Res. 2015, 72, 3–27. [Google Scholar] [CrossRef] [PubMed]
- Du, S.N.N.; Choi, J.A.; McCallum, E.S.; McLean, A.R.; Borowiec, B.G.; Balshine, S.; Scott, G.R. Metabolic implications of exposure to wastewater effluent in bluegill sunfish. Comp. Biochem. Physiol. C-Toxicol. Pharmacol. 2019, 224, 10. [Google Scholar] [CrossRef] [PubMed]
- McCallum, E.S.; Nikel, K.E.; Mehdi, H.; Du, S.N.N.; Bowman, J.E.; Midwood, J.D.; Kidd, K.A.; Scott, G.R.; Balshine, S. Municipal wastewater effluent affects fish communities: A multi-year study involving two wastewater treatment plants. Environ. Pollut. 2019, 252, 1730–1741. [Google Scholar] [CrossRef] [PubMed]
- Simmons, D.B.D.; McCallum, E.S.; Balshine, S.; Chandramouli, B.; Cosgrove, J.; Sherry, J.P. Reduced anxiety is associated with the accumulation of six serotonin reuptake inhibitors in wastewater treatment effluent exposed goldfish Carassius Auratus. Sci. Rep. 2017, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Brooks, B.W.; Chambliss, C.K.; Stanley, J.K.; Ramirez, A.; Banks, K.E.; Johnson, R.D.; Lewis, R.J. Determination of select antidepressants in fish from an effluent-dominated stream. Environ. Toxicol. Chem. 2005, 24, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Huerta, B.; Rodriguez-Mozaz, S.; Lazorchak, J.; Barcelo, D.; Batt, A.; Wathen, J.; Stahl, L. Presence of pharmaceuticals in fish collected from urban rivers in the U.S. EPA 2008–2009 National Rivers and Streams Assessment. Sci. Total Environ. 2018, 634, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Giang, P.T.; Burkina, V.; Sakalli, S.; Schmidt-Posthaus, H.; Rasmussen, M.K.; Randak, T.; Grabic, R.; Grabicova, K.; Fedorova, G.; Koba, O.; et al. Effects of multi-component mixtures from sewage treatment plant effluent on common carp (Cyprinus carpio) under fully realistic condition. J. Environ. Manag. 2019, 63, 466–484. [Google Scholar] [CrossRef] [PubMed]
- Sakalli, S.; Giang, P.T.; Burkina, V.; Zamaratskaia, G.; Rasmussen, M.K.; Bakal, T.; Tilami, S.K.; Sampels, S.; Kolarova, J.; Grabic, R.; et al. The effects of sewage treatment plant effluents on hepatic and intestinal biomarkers in common carp (Cyprinus carpio). Sci. Total Environ. 2018, 635, 1160–1169. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 2014, 473, 619–641. [Google Scholar] [CrossRef] [PubMed]
- Couto, C.F.; Lange, L.C.; Amaral, M.C.S. Occurrence, fate and removal of pharmaceutically active compounds (PhACs) in water and wastewater treatment plants—A review. J. Water Process. Eng. 2019, 32, 100927. [Google Scholar] [CrossRef]
- Golovko, O.; Kumar, V.; Fedorova, G.; Randak, T.; Grabic, R. Seasonal changes in antibiotics, antidepressants/psychiatric drugs, antihistamines and lipid regulators in a wastewater treatment plant. Chemosphere 2014, 111, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Archer, E.; Petrie, B.; Kasprzyk-Hordern, B.; Wolfaardt, G.M. The fate of pharmaceuticals and personal care products (PPCPs), endocrine disrupting contaminants (EDCs), metabolites and illicit drugs in a WWTW and environmental waters. Chemosphere 2017, 174, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Wassenaar, P.N.H.; Verbruggen, E.M.J.; Cieraad, E.; Peijnenburg, W.J.G.M.; Vijver, M.G. Variability in fish bioconcentration factors: Influences of study design and consequences for regulation. Chemosphere 2020, 239, 124731. [Google Scholar] [CrossRef] [PubMed]
- Koba, O.; Steinbach, C.; Kroupova, H.K.; Grabicova, K.; Randak, T.; Grabic, R. Investigation of diltiazem metabolism in fish using a hybrid quadrupole/orbital trap mass spectrometer. RCM 2016, 30, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Steinbach, C.; Grabic, R.; Fedorova, G.; Koba, O.; Golovko, O.; Grabicova, K.; Kroupova, H.K. Bioconcentration, metabolism and half-life time of the human therapeutic drug diltiazem in rainbow trout. Chemosphere 2016, 144, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Grabicova, K.; Vojs Staňová, A.; Koba Ucun, O.; Borik, A.; Randak, T.; Grabic, R. Development of a robust extraction procedure for the HPLC-ESI-HRPS determination of multi-residual pharmaceuticals in biota samples. Analytica Chimica Acta 2018, 1022, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Koba, O.; Grabicova, K.; Cerveny, D.; Turek, J.; Kolarova, J.; Randak, T.; Zlabek, V.; Grabic, R. Transport of pharmaceuticals and their metabolites between water and sediments as a further potential exposure for aquatic organisms. J. Hazard. Mater. 2018, 342, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Rubio, J.; Rodríguez-Gil, J.L.; Postigo, C.; Mastroianni, N.; López de Alda, M.; Barceló, D.; Valcárcel, Y. Psychoactive pharmaceuticals and illicit drugs in coastal waters of North-Western Spain: Environmental exposure and risk assessment. Chemosphere 2019, 224, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Burkina, V.; Zlabek, V.; Zamaratskaia, G. Effects of pharmaceuticals present in aquatic environment on Phase I metabolism in fish. Environ. Toxicol. Pharmacol. 2015, 40, 430–444. [Google Scholar] [CrossRef] [PubMed]
- Burkina, V.; Rasmussen, M.K.; Pilipenko, N.; Zamaratskaia, G. Comparison of xenobiotic-metabolising human, porcine, rodent, and piscine cytochrome P450. Toxicology 2017, 375, 10–27. [Google Scholar] [CrossRef] [PubMed]
- Dubreil, E.; Sczubelek, L.; Burkina, V.; Zlabek, V.; Sakalli, S.; Zamaratskaia, G.; Hurtaud-Pessel, D.; Verdon, E. In vitro investigations of the metabolism of Victoria pure blue BO dye to identify main metabolites for food control in fish. Chemosphere 2020, 238, 124538. [Google Scholar] [CrossRef] [PubMed]
- Zlabek, V.; Burkina, V.; Borrisser-Pairó, F.; Sakalli, S.; Zamaratskaia, G. Phase I metabolism of 3-methylindole, an environmental pollutant, by hepatic microsomes from carp (Cyprinus carpio) and rainbow trout (Oncorhynchus mykiss). Chemosphere 2016, 150, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.J.; Bai, A.; Kulkarni, A.A.; Moghaddam, M.F. Efficiency in drug discovery: Liver S9 fraction assay as a screen for metabolic stability. Drug Metab. Lett. 2016, 10, 83–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hazinski, T.A.; Noisin, E.; Hamon, I.; DeMatteo, A. Sheep lung cytochrome P4501A1 (CYP1A1): cDNA cloning and transcriptional regulation by oxygen tension. J. Clin. Invest. 1995, 96, 2083–2089. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-Y.; Iwata, H.; Fujise, Y.; Tanabe, S. Searching for novel CYP members using cDNA library from a minke whale liver. Mar. Environ. Res. 2004, 58, 495–498. [Google Scholar] [CrossRef] [PubMed]
- Loos, R.; Carvalho, R.; António, D.C.; Comero, S.; Locoro, G.; Tavazzi, S.; Paracchini, B.; Ghiani, M.; Lettieri, T.; Blaha, L.; et al. EU-wide monitoring survey on emerging polar organic contaminants in wastewater treatment plant effluents. Water Res. 2013, 47, 6475–6487. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman, C.U., Jr.; Mohan, D. Pharmaceuticals of emerging concern in aquatic systems: Chemistry, occurrence, effects, and removal methods. Chem. Rev. 2019, 119, 3510–3673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, K.; Chiba, K.; Yagi, T.; Shimada, N.; Taniguchi, T.; Horie, T.; Tani, M.; Yamamoto, T.; Ishizaki, T.; Kuroiwa, Y. Identification of cytochrome P450 isoforms involved in citalopram N-demethylation by human liver microsomes. J. Pharm. Exp. Ther. 1997, 280, 927–933. [Google Scholar]
- Rochat, B.; Amey, M.; Gillet, M.; Meyer, U.A.; Baumann, P. Identification of three cytochrome P450 isozymes involved in N-demethylation of citalopram enantiomers in human liver microsomes. Pharmacogenetics 1997, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.H.; Lee, J.K.; Cho, D.-Y.; Bae, S.K. Simultaneous determination of metoprolol and its metabolites, α-hydroxymetoprolol and O-desmethylmetoprolol, in human plasma by liquid chromatography with tandem mass spectrometry: Application to the pharmacokinetics of metoprolol associated with CYP2D6 genotypes. J. Sep. Sci. 2014, 37, 1256–1264. [Google Scholar] [PubMed]
- Hicks, J.K.; Bishop, J.R.; Sangkuhl, K.; Müller, D.J.; Ji, Y.; Leckband, S.G.; Leeder, J.S.; Graham, R.L.; Chiulli, D.L.; Llerena, A.; et al. Clinical Pharmacogenetics Implementation, C., Clinical pharmacogenetics implementation consortium (CPIC) guideline for CYP2D6 and CYP2C19 genotypes and dosing of selective serotonin reuptake inhibitors. Clin. Pharmacol. Ther. 2015, 98, 127–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, K.; Ishizuka, T.; Shimada, N.; Yoshimura, Y.; Kamijima, K.; Chiba, K. Sertraline N-demethylation is catalyzed by multiple isoforms of human cytochrome P-450 in vitro. Drug Metabol. Dispos. 1999, 27, 763–766. [Google Scholar]
- Obach, R.S.; Cox, L.M.; Tremaine, L.M. Sertraline is metabolized by multiple cytochrome P450 enzymes, monoamine oxidases, and glucuronyl transferases in human: An in vitro study. Drug Metabol. Dispos. 2005, 33, 262–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Connors, K.A.; Du, B.; Fitzsimmons, P.N.; Hoffman, A.D.; Chambliss, C.K.; Nichols, J.W.; Brooks, B.W. Comparative pharmaceutical metabolism by rainbow trout (Oncorhynchus mykiss) liver S9 fractions. Environ. Toxicol. Chem. 2013, 32, 1810–1818. [Google Scholar] [CrossRef] [PubMed]
- Ereshefsky, L.; Dugan, D. Review of the pharmacokinetics, pharmacogenetics, and drug interaction potential of antidepressants: Focus on venlafaxine. Depress. Anxiety 2000, 12, 30–44. [Google Scholar] [CrossRef]
- Burkina, V.; Sakalli, S.; Pilipenko, N.; Zlabek, V.; Zamaratskaia, G. Effect of human pharmaceuticals common to aquatic environments on hepatic CYP1A and CYP3A-like activities in rainbow trout (Oncorhynchus mykiss): An in vitro study. Chemosphere 2018, 205, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Huang, I.J.; Sirotkin, H.I.; McElroy, A.E. Varying the exposure period and duration of neuroactive pharmaceuticals and their metabolites modulates effects on the visual motor response in zebrafish (Danio rerio) larvae. Neurotoxicol. Teratol. 2019, 72, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.L.; Dreier, J.W.; Liu, X.Q.; Ingstrup, K.G.; Mægbæk, M.L.; Munk-Olsen, T.; Christensen, J. Trend of antidepressants before, during, and after pregnancy across two decades-A population-based study. Brain Behav. 2019, 9, e01441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howland, R.H. A question about the potential cardiac toxicity of escitalopram. J. Psychosoc. Nurs. Ment. Health Serv. 2012, 50, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Piña, I.L.; Di Palo, K.E.; Ventura, H.O. Psychopharmacology and cardiovascular disease. J. Am. Coll. Cardiol. 2018, 71, 2346–2359. [Google Scholar] [CrossRef] [PubMed]
- Wierzbinski, P. Citalopram-what you need to know about this proven antidepressant. Psychiatr. Psychol. Klin. 2019, 19, 344–348. [Google Scholar] [CrossRef]
- von Moltke, L.L.; Greenblatt, D.J.; Grassi, J.M.; Granda, B.W.; Venkatakrishnan, K.; Duan, S.X.; Fogelman, S.M.; Harmatz, J.S.; Shader, R.I. Citalopram and desmethylcitalopram in vitro: Human cytochromes mediating transformation, and cytochrome inhibitory effects. Biol. Psychiatry 1999, 46, 839–849. [Google Scholar] [CrossRef]
- Burkina, V.; Sakalli, S.; Zlabek, V.; Zamaratskaia, G. CYP1A1 activity in rainbow trout is inhibited by the environmental pollutant p-cresol. Environ. Toxicol. Pharmacol. 2018, 62, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Szotakova, B.; Baliharova, V.; Lamka, J.; Nozinova, E.; Wsol, V.; Velik, J.; Machala, M.; Neca, J.; Soucek, P.; Susova, S.; et al. Comparison of in vitro activities of biotransformation enzymes in pig, cattle, goat and sheep. Res. Vet. Sci. 2004, 76, 43–51. [Google Scholar] [CrossRef]
- Maté, M.L.; Lifschitz, A.; Sallovitz, J.; Ballent, M.; Muscher, A.S.; Wilkens, M.R.; Schröder, B.; Lanusse, C.; Virkel, G.L. Cytochrome P450 3A expression and function in liver and intestinal mucosa from dexamethasone-treated sheep. J. Vet. Pharmacol. Ther. 2012, 35, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Stuchlíková, L.; Jirásko, R.; Vokřál, I.; Lamka, J.; Špulák, M.; Holčapek, M.; Szotáková, B.; Bártíková, H.; Pour, M.; Skálová, L. Investigation of the metabolism of monepantel in ovine hepatocytes by UHPLC/MS/MS. Anal. Bioanal. Chem. 2013, 405, 1705–1712. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Sakalli, S.; Burkina, V.; Zlabek, V.; Zamaratskaia, G. Effects of acetone, acetonitrile, ethanol, methanol and DMSO on cytochrome P450 in rainbow trout (Oncorhynchus mykiss) hepatic microsomes. Toxicol. Mech. Met. 2015, 25, 501–506. [Google Scholar]
- Hou, R.; Huang, C.; Rao, K.; Xu, Y.; Wang, Z. Characterized in vitro metabolism kinetics of alkyl organophosphate esters in fish liver and intestinal microsomes. Environ. Sci. Technol. 2018, 52, 3202–3210. [Google Scholar] [CrossRef] [PubMed]
- Stiborova, M.; Borek-Dohalska, L.; Aimova, D.; Kotrbova, V.; Kukackova, K.; Janouchova, K.; Rupertova, M.; Ryslava, H.; Hudecek, J.; Frei, E. Oxidation pattern of the anticancer drug ellipticine by hepatic microsomes-similarity between human and rat systems. Gen. Physiol. Biophys. 2006, 25, 245–261. [Google Scholar] [PubMed]
- Jonsson, M.E.; Brunstrom, B.; Ingebrigtsen, K.; Brandt, I. Cell-specific CYP1A expression and benzo[a]pyrene adduct formation in gills of rainbow trout (Oncorhynchus mykiss) following CYP1A induction in the laboratory and in the field. Environ. Toxicol. Chem. 2004, 23, 874–882. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |

| Biomarker | Units | Fish |
|---|---|---|
| EROD | pmol/min/mg | 5.33 ± 1.99 |
| BFCOD | pmol/min/mg | 5.55 ± 3.58 |
| TNBP | + |
| Metabolites Concentration, pmol/min/mg | LOQ 1 Min-Max, pmol/min | ||||
|---|---|---|---|---|---|
| Control (without S9) | Sheep S9 | Fish S9 | BNF 1 Fish S9 | ||
| N-desmethylcitalopram | <LOQ | 2309 | 295 | 1557 | 8–23 |
| Metoprolol acid | <LOQ | 443 | 274 | 81 | 11–27 |
| Norsertraline | <LOQ | 3251 | 405 | 684 | 68–217 |
| O-desmethylvenlafaxine | <LOQ | 823 | <LOQ | <LOQ | 10–24 |
| Incubation Type | Buffer | Pharmaceutical | S9 fraction | Co-Factors | Pharmaceutical Concentration |
|---|---|---|---|---|---|
| Control | + | - | + piscine or ovine | + | - |
| Control | + | - | + piscine or ovine | + | - |
| Control | + | - | + piscine or ovine | + | - |
| Control | + | + | - | + | 2 µM of citalopram or metoprolol or TNBP or sertraline or venlafaxine |
| Test | + | + | + piscine or ovine | + |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burkina, V.; Sakalli, S.; Giang, P.T.; Grabicová, K.; Staňová, A.V.; Zamaratskaia, G.; Zlabek, V. In Vitro Metabolic Transformation of Pharmaceuticals by Hepatic S9 Fractions from Common Carp (Cyprinus carpio). Molecules 2020, 25, 2690. https://doi.org/10.3390/molecules25112690
Burkina V, Sakalli S, Giang PT, Grabicová K, Staňová AV, Zamaratskaia G, Zlabek V. In Vitro Metabolic Transformation of Pharmaceuticals by Hepatic S9 Fractions from Common Carp (Cyprinus carpio). Molecules. 2020; 25(11):2690. https://doi.org/10.3390/molecules25112690
Chicago/Turabian StyleBurkina, Viktoriia, Sidika Sakalli, Pham Thai Giang, Kateřina Grabicová, Andrea Vojs Staňová, Galia Zamaratskaia, and Vladimir Zlabek. 2020. "In Vitro Metabolic Transformation of Pharmaceuticals by Hepatic S9 Fractions from Common Carp (Cyprinus carpio)" Molecules 25, no. 11: 2690. https://doi.org/10.3390/molecules25112690
APA StyleBurkina, V., Sakalli, S., Giang, P. T., Grabicová, K., Staňová, A. V., Zamaratskaia, G., & Zlabek, V. (2020). In Vitro Metabolic Transformation of Pharmaceuticals by Hepatic S9 Fractions from Common Carp (Cyprinus carpio). Molecules, 25(11), 2690. https://doi.org/10.3390/molecules25112690