Expanding the Role of Sub-Exploited DOE-High Energy Extraction and Metabolomic Profiling towards Agro-Byproduct Valorization: The Case of Carotenoid-Rich Apricot Pulp
Abstract
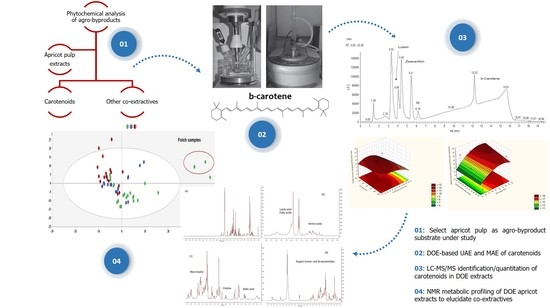
:1. Introduction
2. Results and Discussion
2.1. Extraction Solvent
2.2. Extraction Temperature
2.3. UAE and MAE Optimization Using DOE Models
2.3.1. Screening Design (23 Full Factorial Design)
2.3.2. Response Surface Methodology Models (Box–Behnken Design)
2.3.3. Evaluate the Effects of Extraction Factors under Optimization
Extraction Time
US/MW Power
Solvent/Material Ratio
Optimal Extraction Conditions
2.4. Comparison of Optimized UAE, MAE, and Conventional (Folch) Extractions
2.5. NMR-Based Metabolic Profiling for DOE Apricot Extracts to Elucidate Co-Extractives
3. Materials and Methods
3.1. Reagents and Standards
3.2. Plant Material and Sample Preparation
3.3. Extraction Instrumentation and Processes
3.4. Construction of DOE Models
3.5. Identification and Quantitation of Apricot Pulp Carotenoids by Liquid Chromatography-Photodiode Array-Tandem Mass Spectrometry (LC-PDA-MS/MS)
3.6. NMR Spectroscopy for the Elucidation of Non-Carotenoid Secondary Metabolites of Apricot Extracts
3.6.1. Sample Preparation and NMR Measurements
3.6.2. Data Reduction and Spectral Alignment
3.7. Multivariate Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bijttebier, S.; Van der Auwera, A.; Foubert, K.; Voorspoels, S.; Pieters, L.; Apers, S. Bridging the gap between comprehensive extraction protocols in plant metabolomics studies and method validation. Anal. Chim. Acta 2016, 935, 136–150. [Google Scholar] [CrossRef] [PubMed]
- Fotakis, C.; Tsigrimani, D.; Tsiaka, T.; Lantzouraki, D.Z.; Strati, I.F.; Makris, C.; Tagkouli, D.; Proestos, C.; Sinanoglou, V.J.; Zoumpoulakis, P. Metabolic and antioxidant profiles of herbal infusions and decoctions. Food Chem. 2016, 211, 963–971. [Google Scholar] [CrossRef] [PubMed]
- De Falco, B.; Lanzotti, V. NMR spectroscopy and mass spectrometry in metabolomics analysis of Salvia. Phytochem. Rev. 2018, 17, 951–972. [Google Scholar] [CrossRef]
- Tsiaka, T.; Sinanoglou, V.J.; Zoumpoulakis, P. Extracting Bioactive Compounds From Natural Sources Using Green High-Energy Approaches: Trends and Opportunities in Lab- and Large-Scale Applications. In Ingredients Extraction by Physicochemical Methods in Food; Grumezescu, A.M., Holban, A.M., Eds.; Handbook of Food Bioengineering; Academic Press: Cambridge, MA, USA, 2017; Volume 4, pp. 307–365. ISBN 978-0-12-811521-3. [Google Scholar]
- Acquadro, S.; Appleton, S.; Marengo, A.; Bicchi, C.; Sgorbini, B.; Mandrone, M.; Gai, F.; Peiretti, P.G.; Cagliero, C.; Rubiolo, P. Grapevine Green Pruning Residues as a Promising and Sustainable Source of Bioactive Phenolic Compounds. Molecules 2020, 25, 464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Žlabur, J.Š.; Žutić, I.; Radman, S.; Pleša, M.; Brnčić, M.; Barba, F.J.; Rocchetti, G.; Lucini, L.; Lorenzo, J.M.; Domínguez, R.; et al. Effect of Different Green Extraction Methods and Solvents on Bioactive Components of Chamomile (Matricaria chamomilla L.) Flowers. Molecules 2020, 25, 810. [Google Scholar] [CrossRef] [Green Version]
- Luque de Castro, M.D.; Delgado-Povedano, M.M. Ultrasound: A subexploited tool for sample preparation in metabolomics. Anal. Chim. Acta 2014, 806, 74–84. [Google Scholar] [CrossRef]
- Saini, R.K.; Moon, S.H.; Keum, Y.S. An updated review on use of tomato pomace and crustacean processing waste to recover commercially vital carotenoids. Food Res. Int. 2018, 108, 516–529. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [Green Version]
- Pimentel-Moral, S.; de la Luz Cádiz-Gurrea, M.; Rodríguez-Pérez, C.; Segura-Carretero, A. Recent advances in extraction technologies of phytochemicals applied for the revaluation of agri-food by-products. In Functional and Preservative Properties of Phytochemicals; Prakash, B., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 209–239. ISBN 978-0-12-818593-3. [Google Scholar]
- Lai, W.T.; Khong, N.M.H.; Lim, S.S.; Hee, Y.Y.; Sim, B.I.; Lau, K.Y.; Lai, O.M. A review: Modified agricultural by-products for the development and fortification of food products and nutraceuticals. Trends Food Sci. Tech. 2017, 59, 148–160. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green extraction of natural products: Concept and principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef] [Green Version]
- Saini, R.K.; Nile, S.H.; Park, S.W. Carotenoids from fruits and vegetables: Chemistry, analysis, occurrence, bioavailability and biological activities. Food Res. Int. 2015, 76, 735–750. [Google Scholar] [CrossRef] [PubMed]
- Campbell, O.E.; Padilla-Zakour, O.I. Phenolic and carotenoid composition of canned peaches (Prunus persica) and apricots (Prunus armeniaca) as affected by variety and peeling. Food Res. Int. 2013, 54, 448–455. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.S. Carotenoid extraction methods: A review of recent developments. Food Chem. 2018, 240, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Shnimizu, M.; Moriwaki, H. Cancer chemoprevention by carotenoids. Molecules 2012, 17, 3202–3242. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Tsiaka, T.; Lantzouraki, D.Z.; Siapi, E.; Sinanoglou, V.J.; Heropoulos, G.A.; Calokerinos, A.C.; Zoumpoulakis, P. Macular carotenoids in lipid food matrices: DOE-based high energy extraction of egg yolk xanthophylls and quantification through a validated APCI(+) LC-MS/MS method. J. Chromatogr. B 2018, 1096, 160–171. [Google Scholar] [CrossRef]
- Belwal, T.; Ezzat, S.M.; Rastrelli, L.; Bhatt, I.D.; Daglia, M.; Baldi, A.; Devkota, H.P.; Orhan, I.E.; Patra, J.K.; Das, G.; et al. A critical analysis of extraction techniques used for botanicals: Trends, priorities, industrial uses and optimization strategies. TrAC Trends Anal. Chem. 2018, 100, 82–102. [Google Scholar] [CrossRef]
- Chan, C.H.; Yusoff, R.; Ngoh, G.-C.; Kung, F.W.-L. Microwave-assisted extractions of active ingredients from plants. J. Chromatogr. A 2011, 1218, 6213–6225. [Google Scholar] [CrossRef]
- Hiranvarachat, B.; Devahastin, S.; Chiewchan, N.; Vijaya Raghavan, G.S. Structural modification by different pretreatment methods to enhance microwave-assisted extraction of β-carotene from carrots. J. Food Eng. 2013, 115, 190–197. [Google Scholar] [CrossRef]
- Mercado-Mercado, G.; Montalvo-González, E.; González-Aguilar, G.A.; Alvarez-Parrilla, E.; Sáyago-Ayerdi, S.G. Ultrasound-assisted extraction of carotenoids from mango (Mangifera indica L. ‘Ataulfo’) by-products on in vitro bioaccessibility. Food Biosci. 2018, 21, 125–131. [Google Scholar] [CrossRef]
- Chuyen, H.V.; Nguyen, M.H.; Roach, P.D.; Golding, J.B.; Parks, S.E. Microwave-assisted extraction and ultrasound-assisted extraction for recovering carotenoids from Gac peel and their effects on antioxidant capacity of the extracts. Food Sci. Nutr. 2018, 6, 189–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, J.; Yang, Q.; Huang, W.; Xiao, Y.; Li, D.; Liu, C. Optimization of trans lutein from pumpkin (Cucurbita moschata) peel by ultrasound-assisted extraction. Food Bioprod. Proc. 2018, 107, 104–112. [Google Scholar] [CrossRef]
- Nayak, B.; Dahmoune, F.; Moussi, K.; Remini, H.; Dairi, S.; Aoun, O.; Khodir, M. Comparison of microwave, ultrasound and accelerated-assisted solvent extraction for recovery of polyphenols from Citrus sinensis peels. Food Chem. 2015, 187, 507–516. [Google Scholar] [CrossRef]
- Carail, M.; Fabiano-Tixier, A.-S.; Meullemiestre, A.; Chemat, F.; Caris-Veyrat, C. Effects of high power ultrasound on all-E-β-carotene, newly formed compounds analysis by ultra-high-performance liquid chromatography-tandem mass spectrometry. Ultrason. Sonochem. 2015, 26, 200–209. [Google Scholar] [CrossRef]
- Zaghdoudi, K.; Pontvianne, S.; Framboisier, X.; Achard, M.; Kudaibergenova, R.; Ayadi-Trabelsi, M.; Kalthoum-Cherif, J.; Vanderesse, R.; Frochot, C.; Guiavarc’h, Y. Accelerated solvent extraction of carotenoids from: Tunisian Kaki (Diospyros kaki L.), peach (Prunus persica L.) and apricot (Prunus armeniaca L.). Food Chem. 2015, 184, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Leong, S.Y.; Oey, I. Effects of processing on anthocyanins, carotenoids and vitamin C in summer fruits and vegetables. Food Chem. 2012, 133, 1577–1587. [Google Scholar] [CrossRef]
- Botoran, O.R.; Ionete, R.E.; Miricioiu, M.G.; Costinel, D.; Radu, G.L.; Popescu, R. Amino Acid Profile of Fruits as Potential Fingerprints of Varietal Origin. Molecules 2019, 24, 4500. [Google Scholar] [CrossRef] [Green Version]
- Andreou, V.; Strati, I.F.; Fotakis, C.; Liouni, M.; Zoumpoulakis, P.; Sinanoglou, V.J. Herbal distillates: A new era of grape marc distillates with enriched antioxidant profile. Food Chem. 2018, 253, 171–178. [Google Scholar] [CrossRef]
- Sochor, J.; Skutkova, H.; Babula, P.; Zitka, O.; Cernei, N.; Rop, O.; Krska, B.; Adam, V.; Provazník, I.; Kizek, R. Mathematical evaluation of the amino acid and polyphenol content and antioxidant activities of fruits from different apricot cultivars. Molecules 2011, 16, 7428–7457. [Google Scholar] [CrossRef] [Green Version]
- Carrera, C.; Ruiz-Rodríguez, A.; Palma, M.; Barroso, C.G. Ultrasound-assisted extraction of amino acids from grapes. Ultrason. Sonochem. 2015, 22, 499–505. [Google Scholar] [CrossRef]
- Rebecca, O.P.S.; Boyce, A.N.; Somasundram, C. Isolation and identification of myo-inositol crystals from dragon fruit (Hylocereus polyrhizus). Molecules 2012, 17, 4583–4594. [Google Scholar] [CrossRef] [PubMed]
- Tetik, N.; Yüksel, E. Ultrasound-assisted extraction of d-pinitol from carob pods using Response Surface Methodology. Ultrason. Sonochem. 2014, 21, 860–865. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Aceituno, L.; García-Sarrió, M.J.; Alonso-Rodriguez, B.; Ramos, L.; Sanz, M.L. Extraction of bioactive carbohydrates from artichoke (Cynara scolymus L.) external bracts using microwave assisted extraction and pressurized liquid extraction. Food Chem. 2016, 196, 1156–1162. [Google Scholar] [CrossRef] [PubMed]
- Ramandi, N.F.; Ghassempour, A.; Najafi, N.M.; Ghasemi, E. Optimization of ultrasonic assisted extraction of fatty acids from Borago Officinalis L. flower by central composite design. Arab. J. Chem. 2017, 10, S23–S27. [Google Scholar] [CrossRef] [Green Version]
- Esquivel-Hernández, D.A.; Rodríguez-Rodríguez, J.; Rostro-Alanis, M.; Cuéllar-Bermúdez, S.P.; Mancera-Andrade, E.I.; Núñez-Echevarría, J.E.; García-Pérez, J.S.; Chandra, R.; Parra-Saldívar, R. Advancement of green process through microwave-assisted extraction of bioactive metabolites from Arthrospira Platensis and bioactivity evaluation. Bioresour. Technol. 2017, 224, 618–629. [Google Scholar] [CrossRef]
- Yonemori, K.M.; Lim, U.; Koga, K.R.; Wilkens, L.R.; Au, D.; Boushey, C.J.; Le Marchand, L.; Kolonel, L.N.; Murphy, S.P. Dietary choline and betaine intakes vary in an adult multiethnic population. J. Nutr. 2013, 143, 894–899. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Santos, B.; Rodríguez-Miranda, J.; Herman-Lara, E.; Torruco-Uco, J.G.; Carmona-García, R.; Juárez-Barrientos, J.M.; Chávez-Zamudio, R.; Martínez-Sánchez, C.E. Effect of oil extraction assisted by ultrasound on the physicochemical properties and fatty acid profile of pumpkin seed oil (Cucurbita pepo). Ultrason. Sonochem. 2016, 31, 429–436. [Google Scholar] [CrossRef]
- Kim, H.-R.; Kim, I.-D.; Dhungana, S.K.; Kim, M.-O.; Shin, D.-H. Comparative assessment of physicochemical properties of unripe peach (Prunus persica) and Japanese apricot (Prunus mume). Asian Pac. J. Trop. Biomed. 2014, 4, 97–103. [Google Scholar] [CrossRef] [Green Version]
- Bae, H.; Yun, S.K.; Yoon, I.K.; Nam, E.Y.; Kwon, J.H.; Jun, J.H. Assessment of organic acid and sugar composition in apricot, plumcot, plum, and peach during fruit development. J. Appl. Bot. Food Qual. 2014, 87. [Google Scholar]
- Ren, B.; Chen, C.; Li, C.; Fu, X.; You, L.; Liu, R.H. Optimization of microwave-assisted extraction of Sargassum thunbergii polysaccharides and its antioxidant and hypoglycemic activities. Carbohydr. Polym. 2017, 173, 192–201. [Google Scholar] [CrossRef]
- Wang, K.; Li, M.; Wen, X.; Chen, X.; He, Z.; Ni, Y. Optimization of ultrasound-assisted extraction of okra (Abelmoschus esculentus (L.) Moench) polysaccharides based on response surface methodology and antioxidant activity. Int. J. Biol. Macromol. 2018, 114, 1056–1063. [Google Scholar] [CrossRef] [PubMed]
- Lahaye, M.; Falourd, X.; Quemener, B.; Devaux, M.-F.; Audergon, J.-M. Histological and cell wall polysaccharide chemical variability among apricot varieties. LWT Food Sci.Technol. 2014, 58, 486–496. [Google Scholar] [CrossRef]
- Mushtaq, M.Y.; Choi, Y.H.; Verpoorte, R.; Wilson, E.G. Extraction for metabolomics: Access to the metabolome. Phytochem. Anal. 2014, 25, 291–306. [Google Scholar] [CrossRef] [PubMed]
- Cajka, T.; Fiehn, O. Comprehensive analysis of lipids in biological systems by liquid chromatography-mass spectrometry. TrAC-Trend Anal. Chem. 2014, 61, 192–206. [Google Scholar] [CrossRef] [Green Version]
- Rivera, S.M.; Christou, P.; Canela-Garayoa, R. Identification of carotenoids using mass spectrometry. Mass Spectrom. Rev. 2014, 33, 353–372. [Google Scholar] [CrossRef] [Green Version]
- Van Breemen, R.B.; Dong, L.; Pajkovic, N.D. Atmospheric Pressure Chemical Ionization Tandem Mass Spectrometry of Carotenoids. Int. J. Mass. Spectrom. 2012, 312, 163–172. [Google Scholar] [CrossRef] [Green Version]
- Abraham, J. International Conference On Harmonisation Of Technical Requirements For Registration Of Pharmaceuticals For Human Use. In Handbook of Transnational Economic Governance Regimes; Tietje, C., Brouder, A., Eds.; Brill|Nijhoff: Leiden, The Netherlands, 2010; ISBN 978-90-04-18156-4. [Google Scholar]
- Nimalaratne, C.; Wu, J.; Schieber, A. Egg Yolk Carotenoids: Composition, Analysis, and Effects of Processing on Their Stability. In Carotenoid Cleavage Products; American Chemical Society: Washington, DC, USA, 2013; Volume 1134, pp. 219–225. ISBN 978-0-8412-2778-1. [Google Scholar]
- Gupta, P.; Sreelakshmi, Y.; Sharma, R. A rapid and sensitive method for determination of carotenoids in plant tissues by high performance liquid chromatography. Plant Methods 2015, 11, 5. [Google Scholar] [CrossRef] [Green Version]
- Filntisi, A.; Fotakis, C.; Asvestas, P.; Matsopoulos, G.K.; Zoumpoulakis, P.; Cavouras, D. Automated metabolite identification from biological fluid 1H NMR spectra. Metabolomics 2017, 13, 146. [Google Scholar] [CrossRef]
- Marincola, F.C.; Dessì, A.; Pattumelli, M.G.; Corbu, S.; Ossicini, C.; Ciccarelli, S.; Agostino, R.; Mussap, M.; Fanos, V. (1)H NMR-based urine metabolic profile of IUGR, LGA, and AGA newborns in the first week of life. Clin. Chim. Acta 2015, 451, 28–34. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. Update on natural food pigments-A mini-review on carotenoids, anthocyanins, and betalains. Food Res. Int. 2019, 124, 200–205. [Google Scholar] [CrossRef]
- Jacobson, S.W.; Carter, R.C.; Molteno, C.D.; Stanton, M.E.; Herbert, J.S.; Lindinger, N.M.; Lewis, C.E.; Dodge, N.C.; Hoyme, H.E.; Zeisel, S.H.; et al. Efficacy of Maternal Choline Supplementation During Pregnancy in Mitigating Adverse Effects of Prenatal Alcohol Exposure on Growth and Cognitive Function: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Alcohol Clin. Exp. Res. 2018, 42, 1327–1341. [Google Scholar] [CrossRef] [PubMed]
- Pera, B.; Krumsiek, J.; Assouline, S.E.; Marullo, R.; Patel, J.; Phillip, J.M.; Román, L.; Mann, K.K.; Cerchietti, L. Metabolomic Profiling Reveals Cellular Reprogramming of B-Cell Lymphoma by a Lysine Deacetylase Inhibitor through the Choline Pathway. EBioMedicine 2018, 28, 80–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeisel, S.H.; da Costa, K.-A. Choline: An essential nutrient for public health. Nutr. Rev. 2009, 67, 615–623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monastra, G.; Unfer, V.; Harrath, A.H.; Bizzarri, M. Combining treatment with myo-inositol and D-chiro-inositol (40:1) is effective in restoring ovary function and metabolic balance in PCOS patients. Gynecol. Endocrinol. 2017, 33, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Laganà, A.S.; Garzon, S.; Casarin, J.; Franchi, M.; Ghezzi, F. Inositol in Polycystic Ovary Syndrome: Restoring Fertility through a Pathophysiology-Based Approach. Trends Endocrinol. Metab. 2018, 29, 768–780. [Google Scholar] [CrossRef] [PubMed]
- Brunst, K.J.; Ryan, P.H.; Altaye, M.; Yolton, K.; Maloney, T.; Beckwith, T.; LeMasters, G.; Cecil, K.M. Myo-inositol mediates the effects of traffic-related air pollution on generalized anxiety symptoms at age 12 years. Environ. Res. 2019, 175, 71–78. [Google Scholar] [CrossRef]
- Garcia-Cervera, E.; Figueroa-Valverde, L.; Pool Gomez, E.; Rosas-Nexticapa, M.; Lenin, H.-H.; Virginia, M.-A.; Perla, P.-G.; Regina, C.-C. Biological activity exerted by omega-3 fatty acids on body mass index, glucose, total cholesterol and blood pressure in obese children. Integr. Obesity Diabetes 2018, 4. [Google Scholar] [CrossRef]
- Innes, J.K.; Calder, P.C. Omega-6 fatty acids and inflammation. Prostaglandins Leukot. Ess. Fat. Acids 2018, 132, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Rogero, M.; Calder, P. Obesity, Inflammation, Toll-Like Receptor 4 and Fatty Acids. Nutrients 2018, 10, 432. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Not available. |











| Extraction Parameters | Optimal Values | |
|---|---|---|
| UAE | MAE | |
| Extraction solvent (v/v) | Methanol:chloroform 1:1 | Ethanol |
| Extraction time (min) | 10 | 20 |
| US/MW power (W) | 600 | 120 |
| Solvent/material ratio (mL g−1) | 35 | 45 |
| US pulse sequence (s)/MW ramping time (min) | 15 ON 5 OFF | 0 |
| Extraction yield (mg of carotenoids 100 g−1 dry sample) (±stdev), n = 3 1 | 11.12 (±0.34) | 19.28 (±0.27) |
| Carotenoids Content (mg 100 g−1 Dry Sample), v = 3 1 | Optimized UAE Extract | Optimized MAE Extract | Folch Extract |
|---|---|---|---|
| β-Carotene | 7.72 (±0.98) | 19.7 (±1.6) | 1.44 (±0.87) |
| Zeaxanthin | 0.71 (±0.33) | 0.66 (±0.25) | 0.020 (±0.041) |
| Lutein | 0.82 (±0.19) | 0.82 (±0.12) | 0.07 (±0.24) |
| Final carotenoid content expressed in mg kg−1 of raw apricot pulp sample (N = 3) 2 | |||
| Average weight (g) of raw apricot pulp samples (±stdev), n = 10 3 | 17.1 (±2.1) | ||
| Average weight (g) of lyophilized apricot pulp samples (±stdev), n = 10 3 | 3.23 (±0.39) | ||
| Average moisture (%) of raw apricot pulp, n = 10 3 | 81.25 | ||
| UAE Extracts | MAE Extracts | Folch Extract | |
| β-Carotene content (mg kg−1 raw apricot pulp) (±stdev) | 14.58 (±0.98) | 37.2 (±1.6) | 2.72 (±0.87) |
| Zeaxanthin content (mg kg−1 raw apricot pulp) (±stdev) | 1.34 (±0.33) | 1.25 (±0.25) | 0.038 (±0.041) |
| Lutein content (mg kg−1 raw apricot pulp) (±stdev) | 1.55 (±0.19) | 1.55 (±0.12) | 0.13 (±0.24) |
| Compounds | 1H Chemical Shift | Peak Multiplicity 1 |
|---|---|---|
| Valine | 0.99, 1.04, 2.28 | (d), (d), (m) |
| Leucine | 0.98, 0.96 | (d, J = 7.5), (d, J = 7.5) |
| Isoleucine | 0.94, 1.01, 1.25, 1.45, 1.96, 3.66 | (t), (d), (m), (m), (m), (m) |
| Alanine | 1.48 | (d) |
| Lysine | 1.61 | (t) |
| Choline | 3.10 | (s) |
| Fatty acids | 1.26 | (m) |
| Myo-inositol | 3.67, 3.78 | (t), (t) |
| Malic acid | 2.68, 2.78 | (dd), (dd) |
| Lactic acid | 1.34 | (d) |
| Formic acid | 8.40 | (s) |
| Fructose | 3.53, 4.04 | (t), (t) |
| Sucrose | 4.18, 5.39 | (d), (d, J = 3.9) |
| Glucose | 5.12 | (d) |
| Xylose | 5.07 | (d) |
| Coded Values | −1 | 0 | +1 |
|---|---|---|---|
| 23 design | |||
| UAE | |||
| Extraction time (X1, min) | 5 | 35 | |
| US power (X2, W) | 375 | 675 | |
| Solvent/material ratio (X3, mL g−1) | 10 | 35 | |
| MAE | |||
| Extraction time (X1, min) | 5 | - | 30 |
| MW power (X2, W) | 70 | - | 200 |
| Solvent/material ratio (X3, mL g−1) | 20 | - | 60 |
| BBD | |||
| UAE | |||
| Extraction time (X1, min) | 10 | 20 | 30 |
| US power (X2, W) | 577 | 622 | 675 |
| Solvent/material ratio (X3, mL g−1) | 25 | 30 | 35 |
| MAE | |||
| Extraction time (X1, min) | 5 | 10 | 20 |
| MW power (X2, W) | 60 | 90 | 130 |
| Solvent/material ratio (X3, mL g−1) | 45 | 65 | 55 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsiaka, T.; Fotakis, C.; Lantzouraki, D.Z.; Tsiantas, K.; Siapi, E.; Sinanoglou, V.J.; Zoumpoulakis, P. Expanding the Role of Sub-Exploited DOE-High Energy Extraction and Metabolomic Profiling towards Agro-Byproduct Valorization: The Case of Carotenoid-Rich Apricot Pulp. Molecules 2020, 25, 2702. https://doi.org/10.3390/molecules25112702
Tsiaka T, Fotakis C, Lantzouraki DZ, Tsiantas K, Siapi E, Sinanoglou VJ, Zoumpoulakis P. Expanding the Role of Sub-Exploited DOE-High Energy Extraction and Metabolomic Profiling towards Agro-Byproduct Valorization: The Case of Carotenoid-Rich Apricot Pulp. Molecules. 2020; 25(11):2702. https://doi.org/10.3390/molecules25112702
Chicago/Turabian StyleTsiaka, Thalia, Charalambos Fotakis, Dimitra Z. Lantzouraki, Konstantinos Tsiantas, Eleni Siapi, Vassilia J. Sinanoglou, and Panagiotis Zoumpoulakis. 2020. "Expanding the Role of Sub-Exploited DOE-High Energy Extraction and Metabolomic Profiling towards Agro-Byproduct Valorization: The Case of Carotenoid-Rich Apricot Pulp" Molecules 25, no. 11: 2702. https://doi.org/10.3390/molecules25112702
APA StyleTsiaka, T., Fotakis, C., Lantzouraki, D. Z., Tsiantas, K., Siapi, E., Sinanoglou, V. J., & Zoumpoulakis, P. (2020). Expanding the Role of Sub-Exploited DOE-High Energy Extraction and Metabolomic Profiling towards Agro-Byproduct Valorization: The Case of Carotenoid-Rich Apricot Pulp. Molecules, 25(11), 2702. https://doi.org/10.3390/molecules25112702