Pharmacological Evaluation of Artemisia cina Crude CO2 Subcritical Extract after the Removal of Santonin by Means of High Speed Countercurrent Chromatography
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
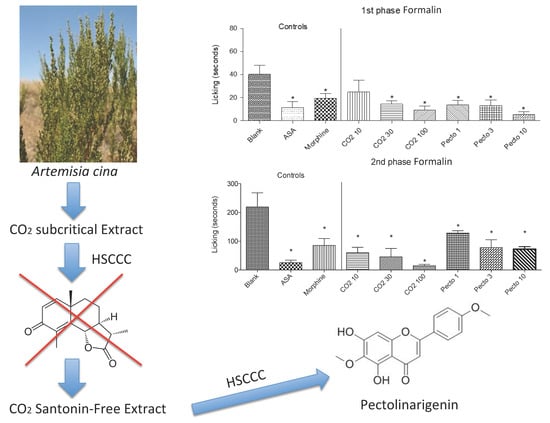
2.2. Plant Material and Extraction
2.3. High Speed Countercurrent Chromatography (HSCCC) Separation Procedure
2.4. Thin Layer Chromatography and Choice of HSCCC System
2.5. Preparation of Santonin-Free Extract (SFCO2E)
2.6. Purification of Isolated Compound
2.7. NMR Identification
2.8. Animals
2.9. Antinociceptive and Anti-Inflammatory Activities In Vivo
2.9.1. Formalin-Induced Licking Response
2.9.2. Thermal-Induced Nociception Model Using a Hot Plate
2.9.3. Capsaicin-Induced Nociception
2.9.4. Glutamate-Induced Nociception
2.9.5. Carrageenan-Induced Cell Migration Using the Subcutaneous Air Pouch (SAP) Model
2.10. Statistical Analysis
3. Results
3.1. Phytochemistry
3.1.1. Isolation of the Pectolinarigenin
3.1.2. Structural Elucidation
3.2. Pharmacology
3.2.1. Nociception-Induced Model Using Formalin
3.2.2. Capsaicin-Induced Nociception
3.2.3. Glutamate-Induced Nociception
3.2.4. Thermal-Induced Nociception Model Using a Hot Plate
3.2.5. Carrageenan-Induced Cell Migration Using Subcutaneous Air Pouch (SAP) Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tan, R.X.; Zheng, W.F.; Tang, H.Q. Biologically active substances from the genus Artemisia. Planta Med. 1998, 64, 295–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bora, K.S.; Shama, A. The Genus Artemisia: A Comprehensive Review. Pharm. Biol. 2011, 49, 101–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abad, M.J.; Bedoya, L.M.; Apaza, L.; Bermejo, P. The Artemisia, L. Genus: A Review of Bioactive Essential Oils. Molecules 2012, 17, 2542–2566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woerdenbag, H.J.; De Smet, P.A.G.M.; Scheffer, J.J.C. Artemisia Cina. In Adverse Effects of Herbal Drugs; De Smet, P.A.G.M., Keller, K., Hansel, R., Chandler, R.F., Eds.; Springer: Berlin/Heidelberg, Germany, 1997; Volume 3, pp. 15–20. [Google Scholar]
- Vardanian, S.A. Phytotherapy of bronchial asthma in medieval Armenian medicine. Ter. Arkh. 1978, 50, 133–136. [Google Scholar] [PubMed]
- Morsy, T.A.; Mazyad, S.A.M.; El-Sharawy, I.M.A. The larvicidal activity of solvent extracts of three medicinal plants against third instar larvae of Chrysomya albiceps. J. Egypt. Soc. Parasitol. 1998, 28, 699–709. [Google Scholar] [PubMed]
- Grange, J.M.; Davey, R.W. Detection of antituberculosis activity of plant extracts. J. Appl. Microbiol. 1990, 68, 587–591. [Google Scholar]
- Asanova, Z.K.; Suleimenov, E.M.; Atazhanova, G.A.; Dembitskii, A.D.; Pak, R.N.; Dar, A.; Adekenov, S.M. Biological activity of 1, 8-cineole from Levant wormwood. Pharm. Chem. J. 2003, 37, 30–32. [Google Scholar] [CrossRef]
- Mannan, A.; Ahmed, I.; Arshad, W.; Asim, M.F.; Qureshi, R.A.; Hussain, I.; Mirza, B. Survey of artemisinin production by diverse Artemisia species in northern Pakistan. Malar. J. 2010, 9, 310. [Google Scholar] [CrossRef] [Green Version]
- Woerdenbag, H.J.; Pras, N. Artemisia, analysis and quality control. In Artemisia; Wright, C., Ed.; Taylor & Francis: London, UK, 2002; p. 56. [Google Scholar]
- Miraldi, E.; Ferri, S.; Franchi, G.G. Santonin: A New Method of Extraction from, and Quantitative Determination in Artemisia caerulescens ssp. cretacea (Fiori) Br.-Catt. & Gubell. by High-Performance Liquid Chromatography. Phytochem. Anal. 1998, 9, 296–298. [Google Scholar]
- Qazilbash, N.A. Santonin—Its detection and estimation. J. Pharm. Pharmacol. 1951, 3, 105–111. [Google Scholar] [CrossRef]
- Singh, B.; Srivastava, J.S.; Khosa, R.L.; Singh, U.P. Individual and Combined Effects of Berberineand Santonin on Spore Germination of Some Fungi. Folia Microbiol. 2001, 46, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Martín, M.L.; Morán, A.; Carrón, R.; Montero, M.J.; San Román, L. Antipyretic activity of α- and β-santonin. J. Ethnopharmacol. 1988, 23, 285–290. [Google Scholar] [PubMed]
- Al-Harbi, M.M.; Qureshi, S.; Ahmed, M.M.; Raza, M.; Miana, G.A.; Shah, A.H. Studies on the Antiinflammatory, Antipyretic and Analgesic Activities of Santonin. Jpn. J. Pharmacol. 1994, 64, 135–139. [Google Scholar] [CrossRef] [Green Version]
- Oettingen, W.F. Comparison of various lactones with santonin. I. Studies of chemical constitution and pharmacological action. J. Pharmacol. Exp. Ther. 1929, 36, 335–354. [Google Scholar]
- Tyler, V.E.; Brady, L.R.; Robbers, J.E. Pharmacognosy, 9th ed.; Lea & Febiger: Philadelphia, PA, USA, 1988; p. 75. [Google Scholar]
- Khares, C.P. Artemisia maritima Linn. In Indian Medicinal Plants: An Illustrated Dictionary; Khares, C.P., Ed.; Springer: Cham, Switzerland, 2007; p. 64. [Google Scholar]
- Arnold, W.N. Vincent van Gogh: Chemicals, Crises, and Creativity; Birkhäuser: Boston, MA, USA, 1992; p. 228. [Google Scholar]
- Grant, W.M.; Schuman, J.S. Encyclopaedia of Chemicals, Drugs, Plants, Toxins, and Venoms. In Toxicology of the Eye: Effects on the Eyes and Visual System from Chemicals, Drugs, Metals and Minerals, Plants, Toxins, and Venoms; Also, Systemic Side Effects from Eye Med, 4th ed.; Charles C Thomas: Springfield, IL, USA, 1993; p. 1253. [Google Scholar]
- Sakipova, Z.; Wong, N.S.H.; Bekezhanova, T.; Sadykova, A.; Shukiberkova, A.; Boylan, F. Quantification of santonin in eight species of Artemisia from Kazakhstan by means of HPLC-UV: Method development and validation. PLoS ONE 2017, 12, e0173714. [Google Scholar] [CrossRef] [Green Version]
- Matheus, M.E.; Berrondo, L.F.; Vieitas, E.C.; Menezes, S.F.; Fernandes, P.D. Evaluation of the antinociceptive properties from Brillantaisia palisotii Lindau stems extracts. J. Ethnopharmacol. 2005, 102, 377–381. [Google Scholar] [CrossRef]
- Sahley, T.L.; Berntson, G.G. Antinociceptive effects of central and systemic administration of nicotine in the rat. Psychopharmacology 1979, 65, 279–283. [Google Scholar] [CrossRef]
- Sakurada, T.; Wako, K.; Sugiyama, A.; Sakurada, C.; Tan-No, K.; Kisara, K. Involvement of spinal NMDA receptors in capsaicin-induced nociception. Pharmacol. Biochem. Behav. 1998, 59, 339–345. [Google Scholar] [CrossRef]
- Beirith, A.; Santos, A.R.; Calixto, J.B. CalixtoMechanisms underlying the nociception and paw oedema caused by injection of glutamate into the mouse paw. Brain Res. 2002, 924, 219–228. [Google Scholar] [CrossRef]
- Raymundo, J.L.R.P.; Guilhon, C.C.; Alviano, D.S.; Matheus, M.E.; Antoniolli, A.; Cavalcanti, S.C.H.; Alves, P.B.; Alviano, C.S.; Fernandes, P.D. Characterisation of the anti-inflammatory and antinociceptive activities of the Hyptis pectinata(L.) Poit essential oil. J. Ethnopharmacol. 2011, 134, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Hase, T.; Ohtani, K.; Kasai, R.; Yamazaki, K.; Picheansoonthon, C. Revised structure for hortensin, a flavonoid from Milingtonia hortensis. Phytochemistry 1995, 40, 287–290. [Google Scholar] [CrossRef]
- Segueni, N.; Zellagui, A.; Moussaoui, F.; Labouel, M.; Rhouati, S. Flavonoids from Algerian Propolis. Arab. J. Chem. 2016, 9, s425–s428. [Google Scholar] [CrossRef] [Green Version]
- Ryakhovskaya, T.V.; Sapko, O.A.; Manadilova, A.M.; Ushbaeva, G.G.; Bokaeva, S.S. Polyphenols of sagebrush and biological activity of its phenolic complex. In Proceedings of the FECS International Conference of Chemistry and Biotechnology of Biological Active Natural Products, Sofia, Bulgaria, 16–21 September 1985; Volume 4, pp. 380–384. [Google Scholar]
- Khazir, J.; Singh, P.P.; Reddy, D.M.; Hyder, I.; Shafi, S.; Sawant, S.D.; Chashoo, G.; Mahajan, A.; Alam, M.S.; Saxena, A.K.; et al. Synthesis and anticancer activity of novel spiro-isoxazoline andspiro-isoxazolidinederivatives of a-santonin. Eur. J. Med. Chem. 2013, 63, 279–289. [Google Scholar] [CrossRef]
- Cummins, T.R.; Sheets, P.L.; Waxman, S.G. The roles of sodium channels in nociception: Implications for mechanisms of pain. Pain 2007, 131, 243–257. [Google Scholar] [CrossRef] [Green Version]
- Cury, Y.; Picolo, G.; Gutierrez, V.P.; Ferreira, S.H. Pain and analgesia: The dual effect of nitric oxide in the nociceptive system. Nitric Oxide 2011, 25, 243–254. [Google Scholar] [CrossRef] [PubMed]
- White, F.A.; Bhangoo, S.K.; Miller, R.J. Chemokines: Integrators of pain and inflammation. Nat. Rev. Drug Discov. 2005, 4, 834–844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCall, W.D.; Tanner, K.D.; Levine, J.D. Formalin induces biphasic activity in C-fibers in the rat. Neurosci. Lett. 1996, 208, 45–48. [Google Scholar] [CrossRef]
- Bars, D.; Gozariu, M.; Cadden, S.W. Animal Models of Nociception. Pharmacol. Rev. 2001, 53, 597–652. [Google Scholar]
- Dray, A. Inflammatory mediators of pain. Br. J. Anaesth. 1995, 75, 125–131. [Google Scholar] [CrossRef]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, M.M.G.; Radulović, N.S.; Miltojević, A.B.; Boylan, F.; Dias Fernandes, P. Antinociceptive esters of N-methylanthranilic acid: Mechanisms of action in heat-mediated pain. Eur. J. Pharmacol. 2014, 727, 106–114. [Google Scholar] [CrossRef]
- Woolf, C.J.; Salter, M.W. Neuronal Plasticity: Increasing the Gain in Pain. Science 2000, 288, 1765–1768. [Google Scholar] [CrossRef] [PubMed]
- Abbasnezhad, A.A.; Khazdair, M.R.; Kianmehr, K. The role of nitric oxide on the oxytocin induce analgesia in mice. Iran. J. Basic Med. Sci. 2016, 19, 238–244. [Google Scholar]
- Takeuchi, O.; Akira, S. Pattern Recognition Receptors and Inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henson, P.M.; Johnston, R.B. Tissue Injury in Inflammation. Oxidants, Proteinases, and Cationic Proteins. J. Clin. Investig. 1987, 79, 669–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabay, C.; Kushner, I. Acute—Phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 1999, 340, 448–454. [Google Scholar] [CrossRef]
- Aich, A.; Afrin, L.B.; Gupta, K. Mast Cell-Mediated Mechanisms of Nociception. Int. J. Mol. Sci. 2015, 16, 29069–29092. [Google Scholar] [CrossRef]
- Jones, H.R.; Robb, C.T.; Perretti, M.; Rossi, A.G. The role of neutrophils in inflammation resolution. Semin. Immunol. 2016, 28, 137–145. [Google Scholar] [CrossRef]
- Ren, K.; Torres, R. Role of interleukin-1βduring pain and inflammation. Brain Res. Rev. 2009, 60, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.M.; An, J. Cytokines, inflammation and pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Funk, C.D. Prostaglandins and Leukotrienes: Advances in Eicosanoid Biology. Science 2007, 294, 1871–1875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seibert, K.; Zhang, Y.; Leahy, K.; Hauser, S.; Masferrer, J.; Perkins, W.; Lee, L.; Isakson, P. Pharmacological and biochemical demonstration of the role of cyclooxygenase 2 in inflammation and pain. Proc. Natl. Acad. Sci. USA 1994, 91, 12013–12017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boers, M. NSAIDS and selective COX-2 inhibitors: Competition between gastroprotection and cardioprotection. Lancet 2001, 357, 1222–1223. [Google Scholar] [CrossRef]
- Calixto, J.B.; Otuki, M.F.; Santos, A.R. Anti-inflamatory compounds of plant origin. Part, I. Action on arachidonic acid pathway, nitric oxide and nuclear factor κB (NF-κB). Planta Med. 2003, 69, 973–983. [Google Scholar]
- Werz, O. 2 Inhibition of 5-lipoxigenase product synthesis by natural compounds of plant origin. Planta Med. 2007, 73, 1331–1357. [Google Scholar] [CrossRef] [Green Version]
- Schmitz, L.M.; Bacher, S. Novel molecular targets in the search for anti-inflamatory agentes. Phytochem. Rev. 2005, 4, 19–25. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of the compound 1 is not available from the authors. |







| Position | Own Data | Hase et al., 1995 [28] | Segueni et al., 2016 [29] | |||
|---|---|---|---|---|---|---|
| δC, mult. | δH, (J in Hz) | δC, mult. | δH, (J in Hz) | δC, mult. | δH, (J in Hz) | |
| 2 | 164.2 | 163 | 163.3 | |||
| 3 | 103.7 | 6.61, s | 102.8 | 6.86, s | 103.9 | 6.83 |
| 4 | 182.9 | 181.3 | 182.6 | |||
| 4a | 105.6 | 103.9 | 104.1 | |||
| 5-OH | 153.2 | 13.05, s | 153.4 | 13.00, s | 152.2 | 13.01 |
| 6 | 130.6 | 131.2 | 131.3 | |||
| 7-OH | 155.5 | 157 | 152.3 | 10.67 | ||
| 8 | 93.6 | 6.59, s | 94 | 6.63, s | 94.3 | 6.59 |
| 8a | 152.3 | 152.1 | 151.9 | |||
| 1′ | 123.5 | 122.7 | 123.7 | |||
| 4′ | 162.6 | 162 | 160.9 | |||
| 2′/6′ | 128.1 | 7.86, d | 127.9 | 8.03, d | 128.8 | 8.00 |
| 3′/5′ | 114.5 | 7.03, d | 114.3 | 7.12, d | 114.1 | 7.08 |
| MeO-4′ | 55.5 | 3.91, s | 55.3 | 3.77, s | 55.8 | 3.75 |
| MeO-6 | 60.8 | 4.03, s | 59.6 | 3.86, s | 60.4 | 3.87 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakipova, Z.; Giorno, T.B.S.; Bekezhanova, T.; Siu Hai Wong, N.; Shukirbekova, A.; Fernandes, P.D.; Boylan, F. Pharmacological Evaluation of Artemisia cina Crude CO2 Subcritical Extract after the Removal of Santonin by Means of High Speed Countercurrent Chromatography. Molecules 2020, 25, 2728. https://doi.org/10.3390/molecules25122728
Sakipova Z, Giorno TBS, Bekezhanova T, Siu Hai Wong N, Shukirbekova A, Fernandes PD, Boylan F. Pharmacological Evaluation of Artemisia cina Crude CO2 Subcritical Extract after the Removal of Santonin by Means of High Speed Countercurrent Chromatography. Molecules. 2020; 25(12):2728. https://doi.org/10.3390/molecules25122728
Chicago/Turabian StyleSakipova, Zuriyadda, Thais Biondino Sardella Giorno, Tolkyn Bekezhanova, Nikki Siu Hai Wong, Alma Shukirbekova, Patricia Dias Fernandes, and Fabio Boylan. 2020. "Pharmacological Evaluation of Artemisia cina Crude CO2 Subcritical Extract after the Removal of Santonin by Means of High Speed Countercurrent Chromatography" Molecules 25, no. 12: 2728. https://doi.org/10.3390/molecules25122728
APA StyleSakipova, Z., Giorno, T. B. S., Bekezhanova, T., Siu Hai Wong, N., Shukirbekova, A., Fernandes, P. D., & Boylan, F. (2020). Pharmacological Evaluation of Artemisia cina Crude CO2 Subcritical Extract after the Removal of Santonin by Means of High Speed Countercurrent Chromatography. Molecules, 25(12), 2728. https://doi.org/10.3390/molecules25122728