Alterations in Herbage Yield, Antioxidant Activities, Phytochemical Contents, and Bioactive Compounds of Sabah Snake Grass (Clinacanthus Nutans L.) with Regards to Harvesting Age and Harvesting Frequency
Abstract
:1. Introduction
2. Results and Discussions
2.1. Herbal Yield
2.2. Total Phenolic Content (TPC) and Total Flavonoid Contents (TFC)
2.3. Radical Scavenging Activity (DPPH) and Ferric Reducing Power Assay (FRAP)
2.4. Bioactive Compounds (C-Glycosyl Flavone)
2.5. The Correlation between Herbal Yield, Antioxidant Activities, and Identified Polyphenolic Compounds
3. Materials and Methods
3.1. Standards and Chemicals
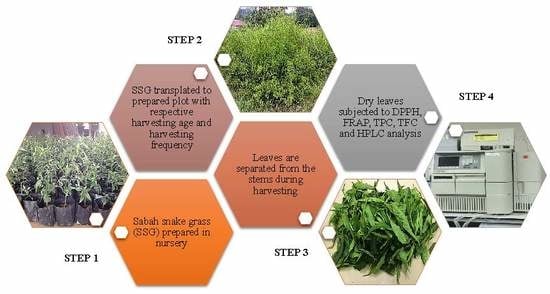
3.2. Planting Materials, Experimental Field, and Treatments
3.3. Extraction Method
3.4. Determination of Total Phenolic Content (TPC)
3.5. Determination of Total Flavonoid Content (TFC)
3.6. DPPH Radical Scavenging Activity Assay
3.7. Ferric Reducing Antioxidant Power Assay (FRAP)
3.8. Separation and Analysis of Bioactive Compounds (C-Glycosyl Flavone) by HPLC
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Source of Variations | d.f | Leaf Dry Weight |
| Block | 4 | 10.29 |
| Harvesting age (HA) | 2 | 3752.88 ** |
| Harvesting frequency (HF) | 2 | 2731.08 ** |
| Interaction (HA × HF) | 4 | 200.75 ** |
| Error | 32 | 3.73 |
| Total | 44 | |
| Contrast | ||
| Linear (HA) | 1 | 2482.52 ** |
| Quadratic (HA) | 1 | 18.94 * |
| Linear (HF) | 1 | 1820.61 ** |
| Quadratic (HF) | 1 | 0.02 |
| * and ** are significantly difference at p < 0.05 and p < 0.01 respectively. | ||
Appendix B
| Source of Variations | d.f | Phytochemicals and Antioxidant Activity | C-glycosyl flavone (µg/g) | |||||||
| DPPH activity (%) | FRAP Activity (Fe (II)/g) | Total Phenolic Content (mg quercetin/g DW) | Total Flavonoid Content (mg GAE/g DW) | Shaftoside | Isoorientin | Orientin | Isovitexin | Vitexin | ||
| Block | 4 | 1.05 | 616.55 | 0.12 | 0.94 | 0.84 | 1.83 | 0.22 | 0.72 | 0.18 |
| Harvesting age (HA) | 2 | 49.85 ** | 10034.29 ** | 95.17 ** | 34.73 ** | 7.03 | 0.82 | 0.03 | 0.28 | 0.17 |
| Harvesting frequency (HF) | 2 | 97.23 ** | 25343.55 ** | 75.63 ** | 22.47 ** | 77.16 * | 14.39 * | 1.71 | 0.94 | 0.59 |
| Interaction (HA × HF) | 4 | 1.38 | 1301.28 * | 3.62 ** | 0.04 | 40.53 | 0.24 | 0.13 | 0.48 | 0.34 |
| Error | 32 | 0.59 | 371.59 | 0.18 | 0.61 | 17.42 | 1.59 | 0.09 | 0.56 | 0.20 |
| Total | 44 | |||||||||
| Contrast | ||||||||||
| Linear (HA) | 1 | 33.20 ** | 6621.36 * | 54.57 * | 22.68 * | 2.55 | 0.355 | 0.01 | 0.25 | 0.11 |
| Quadratic (HA) | 1 | 0.03 | 67.14 | 8.83 ** | 0.50 | 2.16 | 0.00 | 0 | 0.24 | 0.01 |
| Linear (HF) | 1 | 64.77 ** | 16759.20 ** | 50.04 ** | 14.91 ** | 51.10 * | 11.32 * | 1.13 * | 0.33 | 0.11 |
| Quadratic (HF) | 1 | 0.05 | 137.13 | 0.33 * | 0.04 | 0.3 | 2.78 | 0.02 | 0.3 | 0.01 |
| * and ** are significantly difference at p < 0.05 and p < 0.01 respectively. | ||||||||||
References
- Zhang, X.; World Health Organization. Traditional Medicine Strategy. 2002–2005. Available online: http://apps.who.int/medicinedocs/en/d/Js4928e/ (accessed on 22 March 2017).
- Saxena, M.; Saxena, J.; Nema, R.; Singh, D.; Gupta, A. Phytochemistry of medicinal plant. J. Pharmacogn. Phytochem. 2013, 1. [Google Scholar]
- Schijlen, E.G.W.M.; Ric de Vos, C.H.; Tunen, A.J.V.; Bovy, A.G. Modification of flavonoid biosynthesis in crop plants. Phytochemistry 2004, 65, 2631–2648. [Google Scholar] [CrossRef] [PubMed]
- Wanikiat, P.; Panthong, A.; Sujayanon, P.; Yoosok, C.; Rossi, A.G.; Reutrakul, V. The anti-inflammatory effects and the inhibition of neutrophil responsiveness by Barleria lupulina and Clinacanthus nutans extracts. J. Ethnopharmacol. 2007, 116, 234–244. [Google Scholar] [CrossRef]
- Kunsorn, P.; Ruangrungsi, N.; Lipipun, V.; Khanboon, A.; Rungsihirunrat, K. The identities and anti-herpes simplex virus activity of Clinacanthus nutans and Clinacanthus siamensis. Asian Pac. J. Trop. Biomed. 2013, 3, 284–290. [Google Scholar] [CrossRef] [Green Version]
- Shuyprom, A. Chemical composition investigation of the Clinacanthus nutans (Burm. F.) lindau leaves. Master’s Thesis, Suranaree University of Technology, Ratchasima, Thailand, 2004. [Google Scholar]
- Ho, S.Y.; Tiew, W.P.; Madhavan, P.; Abdul Shukkoor, M.S.; Akowuah, G.A. Phytochemical analysis and antibacterial activity of methanolic extract of Clinacanthus nutans leaf. Int. J. Drug Dev. Res. 2013, 5, 349–355. [Google Scholar]
- Wong, F.-C.; Yong, A.-L.; Ong, H.-C.; Chai, T.-T. Evaluation of the antibacterial activities of selected medicinal plants and determination of their phenolic constituents. ScienceAsia 2013, 39, 591–595. [Google Scholar]
- Tinh, T.D.D. Biological activities of Clinacanthus nutans (Burm. F) Lindau Extracts. Ph.D. Thesis, International University HCMC, Ho Chi Minh, Vietnam, 2014. [Google Scholar]
- Abdul Rahim, M.H.; Zakaria, Z.A.; Mohd Sani, M.H. Methanolic extract of Clinacanthus nutans exerts antinociceptive activity via the opioid/nitric oxide-mediated, but cGMP-independent, pathways. Evid.-Based Complementary Altern. Med. 2016, 1–11. [Google Scholar]
- Kittisiripornkul, S.; Witthayasat, K. The Antiinflammatory Action and Toxicological Studies of Extracts from Clinacanthus Nutans. Ph.D. Thesis, Mahidol University, Bangkok, Thailand, 1984. [Google Scholar]
- Satatyavivad, J.; Bunyaprephatsara, N.; Kitisiripornkul, S.; Tanasomwang, W. Analgesic and anti-inflammatory activities of extract of Clinacanthus nutans (Burm.F.) lindau. Thai J. Pharm. Sci. 1996, 3, 7–17. [Google Scholar]
- Uawonggul, N.; Chaveerach, A.; Thammasirirak, S.; Arkaravichien, T.; Chuachan, C.; Daduang, S. Screening of plants acting against Heterometrus laoticus scorpion venomactivity on fibroblast cell lysis. J. Ethnopharmacol. 2006, 103, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Uawonggul, N.; Thammasirirak, S.; Chaveerach, A.; Chuachan, C.; Daduang, J.; Daduang, S. Plant extract activities against the fibroblast cell lysis by honey bee venom. J. Med. Plants Res. 2011, 5, 1978–1986. [Google Scholar]
- Thongharb, C.; Tejasen, P. The effect of Slaed Pang Porn (Clinacanthus nutans) on Thailand cobra venom (Naja naja siamensis). Thai J. Pharm. Sci. 1977, 2, 1057–1063. [Google Scholar]
- Charerntantanakul, W.; Kawaree, R. Effects of medicinal plants extracts on interleukin-10 and tumor necrosis factor alpha gene expressions in porcine peripheral blood mononuclear cells. Chiang Mai Vet. J. 2010, 8, 93–103. [Google Scholar]
- Sriwanthana, B.; Chavalittumrong, P.; Chompuk, L. Effect of Clinacanthus nutans on human cell-mediatedimmune response in vitro. Thail. J. Pharm. Sci. 1996, 20, 261–267. [Google Scholar]
- Tan, C.S.-H.; Ho, C.F.-Y.; Heng, S.-S. Clinacanthus nutans extracts modulate epigenetic link to cytosolic phospholipase A2 expression in SH-SY5Y cells and primary cortical neurons. Neuromolecular Med. 2016, 18, 441–452. [Google Scholar] [CrossRef]
- Tsai, H.-D.; Wu, J.-S.; Kao, M.-H. Clinacanthus nutans protects cortical neurons against hypoxia-induced toxicity by downregulating HDAC1/6. Neuromolecular Med. 2016, 18, 274–282. [Google Scholar] [CrossRef]
- Wu, J.-S.; Kao, M.-H.; Tsai, H.-D. Clinacanthus nutans mitigates neuronal apoptosis and ischemic brain damage through augmenting the C/EBPβ-driven PPAR-γ transcription. Mol. Neurobiol. 2017, 1–14. [Google Scholar]
- Wong, F.C.; Yong, A.L.; Ting, E.P.S. Antioxidant, metal chelating, anti-glucosidase activities and phytochemical analysis of selected tropical medicinal plants. Iran. J. Pharm. Res. 2014, 13, 1407–1413. [Google Scholar]
- Alam, M.A.; Zaidul, I.S.M.; Ghafoor, K. Identification of bioactive compounds with GC–Q-TOF–MS in the extracts from Clinacanthus nutans using subcritical carbon dioxide extraction. Sep. Sci. Technol. (Phila.) 2017, 52, 852–863. [Google Scholar] [CrossRef]
- Lee, S.Y.; Mediani, A.; Nur Ashikin, A.H.; Azliana, A.B.S.; Abas, F. Antioxidant and α-glucosidase inhibitory activities of the leaf and stem of selected traditional medicinal plants. Int. Food Res. J. 2014, 21, 165–172. [Google Scholar]
- Khoo, L.W.; Mediani, A.; Zolkeflee, N.K.Z. Phytochemical diversity of Clinacanthus nutans extracts and their bioactivity correlations elucidated by NMR based metabolomics. Phytochem. Lett. 2015, 14, 123–133. [Google Scholar] [CrossRef]
- Ong, S.L.; Paneerchelvan, S.; Lai, H.Y.; Rao, N.K. In vitro lipase inhibitory effect of thirty-two selected plants in Malaysia. Asian J. Pharm. Clin. Res. 2014, 7, 19–24. [Google Scholar]
- Yuann, J.M.P.; Wang, J.S.; Jian, H.L.; Lin, C.C.; Liang, J.Y. Effects of Clinacanthus nutans (Burm. f) lindau leaf extracts on protection of plasmid DNA from riboflavin photoreaction. Mc-Trans. Biotechnol. 2012, 4, 45–58. [Google Scholar]
- Ng, C.T.; Fong, L.Y.; Tan, J.J.; Rajab, N.F.; Abas, F.; Shaari, K.; Chan, K.M.; Juliana, F.; Yong, Y.K. Water extract of Clinacanthus nutans leaves exhibits in vitro, ex vivo and in vivo antiangiogenic activities in endothelial cell via suppression of cell proliferation. Bmc Complementary Altern. Med. 2018, 18, 210. [Google Scholar] [CrossRef]
- Yong, Y.K.; Tan, J.J.; Teh, S.S.; Mah, S.H.; Ee, G.C.L.; Chiong, H.S.; Ahmad, Z. Clinacanthus nutans extracts are antioxidant with antiproliferative effect on cultured human cancer cell lines. Evid.-Based Complementary Altern. Med. 2013. [Google Scholar] [CrossRef]
- Abd Rahman, N.M.A.N.; Nurliyana, M.Y.; Natasha Nur Afiqah, M.N.F.; Osman, M.A.; Hamid, M.; Mohd Lila, M.A. Antitumor and antioxidant effects of Clinacanthus nutans Lindau in 4 T1 tumor-bearing mice. Bmc Complementary Altern. Med. 2019, 19, 340. [Google Scholar] [CrossRef]
- Amaglo, N.K.; Timpo, G.M.; Ellis, W.O.; Bennett, R.N. Effect of spacing and harvest frequency on the growth and leaf yield of moringa (Moringa oleifera Lam), a leafy vegetable crop. Ghana J. Hortic. 2006, 33–40. [Google Scholar]
- Brum, O.B.; Lopez, S.; Garcia, R.; Andres, S.; Calleja, A. Influence of harvest season, cutting frequency and nitrogen fertilization of mountain meadows on yield, floristic composition and protein content of herbage. Rev. Bras. De Zootec. 2009, 38, 594–604. [Google Scholar] [CrossRef] [Green Version]
- Golpavar, A.R. Determination of the best harvesting times to obtain maximum dry herbage, essential oil and thymol yield in garden thyme (Thymus vulgaris L.). Int. J. Life Sci. Med Res. 2011, 1, 1–4. [Google Scholar]
- Kumar, S.; Kumar, A. Spatial and harvesting influence on growth, yield, quality, and economic potential of Kalmegh (Andrographis paniculata Wall Ex. Nees). J. Agric. Rural Dev. Trop. Subtrop. 2013, 114, 69–76. [Google Scholar]
- Olasoji, J.O.; Aluko, A.O.; Adeniyan, S.O.; Olosunde, A.A.; Okoh, J.O. Effect of time of harvest on physiological maturity and kenaf (Hibiscus canabinus) seed quality. Afr. J. Plant Sci. 2012, 6, 282–289. [Google Scholar] [CrossRef]
- Mequanent, Y.; Ayele, N. The effect of harvesting age on maturity indices of quality parameters of sugarcane varieties at Metahara sugar estate in cool season. J. Agric. Nat. Resour. Sci. 2014, 1, 232–237. [Google Scholar]
- Zigene, Z.D.; Kassahun, B.M.; Ketaw, T.T. Effects of harvesting age and spacing on leaf yield and essential oil yield of rosemary (Rosmarinus officinalis L.). Afr. J. Plant Sci. Biotechnol. 2012, 6, 9–12. [Google Scholar]
- Ghasemzadeh, A.; Nasiri, A.; Jaafar, H.Z.E.; Baghdadi, A.; Ahmad, I. Changes in phytochemical synthesis, chalcone synthase activity and pharmaceutical qualities of Sabah snake grass (Clinacanthus nutans L.) in relation to plant age. Molecules 2014, 19, 17632–17648. [Google Scholar] [CrossRef]
- Hagos, H.; Worku, W.; Takele, A. Effect of drying off period and harvest age on quality and yield of ratoon cane (Saccharium officinarium L.). Adv. Crop Sci. Technol. 2014, 2. [Google Scholar] [CrossRef] [Green Version]
- Gebremeskel, H.; Woldetsadik, K. Effect of plant spacing and harvesting age on growth, biomass and oil yield of rose-scented geranium (Pelargonium Graveolens L. Herit). Basic Res. J. 2015, 4, 94–101. [Google Scholar]
- Maudu, M.; Mudau, F.N.; Mariga, I.K. The effect of pruning on growth and chemical composition of cultivated Bush tea (Athrixia phylicoides D.C). J. Med. Plants Res. 2010, 4, 2353–2358. [Google Scholar] [CrossRef]
- Hue, K.T.; Van, D.T.T.; Ledin, I.; Wredle, E.; Sporndly, E. Effect of harvesting frequency, variety and leaf maturity on nutrient composition, hydrogen cyanide content and cassava foliage yield. Asian-Australas. J. Anim. Sci. 2012, 25, 1691–1700. [Google Scholar] [CrossRef]
- Marasha, N.; Mariga, I.K.; Ngezimana, W.; Mudau, F.N. Effect of pruning on carbohydrate dynamics of herbal and medicinal plant species: Prospects leading to research on the influence of pruning on productivity and biochemical composition of Bush tea (Athrixia phylicoides D.C.). Afr. J. Agric. Res. 2013, 8, 3528–3533. [Google Scholar] [CrossRef] [Green Version]
- Francini, A.; Sebastiani, L. Abiotic stress effects on performance of horticultural crops. Horticulturae 2019, 5, 67. [Google Scholar] [CrossRef] [Green Version]
- Smith, M.R.; Rao, I.M.; Merchant, A. Source-sink relationships in crop plants and their influence on yield development and nutritional quality. Front. Plant Sci. 2018, 9, 1889. [Google Scholar] [CrossRef] [Green Version]
- Du Toit, J.T.; Bryant, J.P.; Frisby, K. Regrowth and palatability of Acacia shoots following pruning by African savanna browsers. Ecology 1990, 71, 149–154. [Google Scholar] [CrossRef]
- Dudonné, S.; Vitrac, X.; Coutière, P.; Woillez, M.; Mérillon, J.-M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jiang, L.; Ye, S.; Ye, Y.; Ren, F. Systematic evaluation of antioxidant capacities of the ethanolic extract of different tissues of jujube (Ziziphus jujuba Mill.) from China. Food Chem. Toxicol. 2010, 48, 1461–1465. [Google Scholar] [CrossRef]
- Qader, S.W.; Abdulla, M.A.; Chua, L.S.; Najim, N.; Zain, M.M.; Hamdan, S. Antioxidant, total phenolic content and cytotoxicity evaluation of selected Malaysian plants. Molecules 2011, 16, 3433–3443. [Google Scholar] [CrossRef] [PubMed]
- Tai, Z.; Cai, L.; Dai, L.; Dong, L.; Wang, M.; Yang, Y.; Cao, Q.; Ding, Z. Antioxidant activity and chemical constituents of edible flower of Sophora viciifolia. Food Chem. 2011, 126, 1648–1654. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.-X.; Chen, J.-W. Commercial quality, major bioactive compound content and antioxidant capacity of 12 cultivars of loquat (Eriobotrya japonica Lindl.) fruits. J. Sci. Food Agric. 2011, 91, 1057–1063. [Google Scholar] [CrossRef]
- Szydłowska-Czerniak, A.; Bartkowiak-Broda, I.; Karlović, I.; Karlovits, G.; Szłyk, E. Antioxidant capacity, total phenolics, glucosinolates and colour parameters of rapeseed cultivars. Food Chem. 2011, 127, 556–563. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 54, 1841–1856. [Google Scholar] [CrossRef]
- Clarke, G.; Ting, K.N.; Wiart, C.; Fry, J. High correlation of 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging, ferric reducing activity potential and total phenolics content indicates redundancy in use of all there assays to screen for antioxidant activity of extracts of plants from the Malaysian rainforest. Antioxidants 2013, 2, 1–10. [Google Scholar]
- Shalaby, E.A.; Shanab, S.M.M. Antioxidant compounds, assays of determination and mode of action. Afr. J. Pharm. Pharmacol. 2013, 7, 528–539. [Google Scholar] [CrossRef]
- Ozgen, M.; Reese, R.N.; Tulio, A.Z., Jr.; Scheerens, J.C.; Miller, A.R. Modified 2,2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) method to measure antioxidant capacity of selected small fruits and comparison to ferric reducing antioxidant power (FRAP) and 2,2′-diphenyl-1-picrylhydrazyl (DPPH) methods. J. Agric. Food Chem. 2006, 54, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Stratil, P.; Klejdus, B.; Kuban, V. Determination of total content of phenlic compounds and their antioxidant activity in vegetables—Evaluation of spectrophotometric methods. J. Agric. Food Chem. 2006, 54, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Halvorsen, B.L.; Blomhoff, R. Validation of a quantitative assay for the total content of lipophilic and hydrophilic antioxidants in foods. Food Chem. 2011, 127, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Raya, K.B.; Ahmad, S.H.; Farhana, S.F.; Mohammad, M.; Tajidin, N.E.; Parvez, A. Changes in phytochemical contents in different parts of Clinacanthus nutans (Burm. f.) Lindau due to storage duration. Bragantia Camp. 2015, 74, 445–452. [Google Scholar] [CrossRef] [Green Version]
- Sulaiman, I.S.C.; Basri, M.; Chan, K.W.; Ashari, S.E.; Masoumi, H.R.F.; Ismail, M. In vitro antioxidant, cytotoxic and phytochemical studies of Clinacanthus nutans Lindau leaf extracts. Afr. J. Pharm. Pharmacol. 2015, 9, 861–874. [Google Scholar] [CrossRef] [Green Version]
- Arullappan, S.; Rajamanickam, P.; Thevar, N.; Kodimani, C.C. In vitro screening of cytotoxic, antimicrobial and antioxidant activities of Clinacanthus nutans (Acanthaceae) leaf extracts. Trop. J. Pharm. Res. 2014, 13, 1455–1461. [Google Scholar] [CrossRef] [Green Version]
- Pannangpetch, P.; Laupattarakasem, P.; Kukongviriyapan, V.; Kukongviriyapan, U.; Kongyingyoes, B.; Aromdee, C. Anti-oxidant activity and protective effect against oxidative haemolysis of Clinacanthus nutans (Burm.f) Lindau. Songklanakarin J. Sci. Technol. 2007, 29, 1–9. [Google Scholar]
- Chelyn, J.L.; Omar, M.H.; Yousuf, N.S.A.M.; Ranggasamy, R.; Wasiman, M.I.; Ismail, Z. Analysis of flavone c-glycosides in the leaves of Clinacanthus nutans (Burm. f.) lindau by HPTLC and HPLC-UV/DAD. Sci. World J. 2014, 6. [Google Scholar] [CrossRef] [Green Version]
- Stalikas, C.D. Extraction, separation, and detection methods for phenolic acids and flavonoids. J. Sep. Sci. 2007, 30, 3268–3295. [Google Scholar] [CrossRef]
- Sliumpaite, I.; Venskutanis, P.R.; Murkovic, M.; Pukalskas, A. Antioxidant properties and polyphenolics composition of common hedge hyssop (Gratiola oficinalis L.). J. Funct. Foods 2013, 5, 1927–1937. [Google Scholar] [CrossRef]
- Roy, J.L.; Huss, B.; Creach, A.; Hawkins, S.; Neutelings, G. Glycosylation is a major regulator of phenylpropanoid availability and biological activity in plants. Front. Plants Sci. 2016, 7, 735. [Google Scholar]
- Rawat, P.; Kumar, M.; Sharan, K.; Chattopadhyay, N.; Maurya, R. Ulmosides A and B: Flavonoid 6-C-glycosides from Ulmus wallichiana, stimulating osteoblast differentiation assessed by alkaline phosphatase. Bioorg. Med. Chem. Lett. 2009, 19, 4684–4687. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liang, Z.; Liao, X.; Zhou, C.; Xie, Z.; Zhu, S.; Wei, G.; Huang, Y. Identification of C-glycosyl flavones by high performance liquid chromatography electrospray ionization mass spectrometry and quantification of five main C-glycosyl flavones in Flickingeria fimbriata. Bmc Chem. 2019, 13, 94. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.Z.; Harnly, J.M. A screening method for the identification of glycosylated flavonoids and other phenolic compounds using a standard analytical approach for all plant materials. J. Agric. Food Chem. 2007, 55, 1084–1096. [Google Scholar] [CrossRef] [Green Version]
- Cheynier, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant phenolics: Recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20. [Google Scholar] [CrossRef]
- Mierziak, J.; Kostyn, K.; Kulma, A. Flavonoids as important molecules of plant interactions with the environment. Molecules 2014, 19, 16240–16265. [Google Scholar] [CrossRef]
- Holbein, J.; Grundler, F.M.; Siddique, S. Plant basal resistance to nematodes: An update. J. Exp. Bot. 2016, 67, 2049–2061. [Google Scholar] [CrossRef] [Green Version]
- Ishihara, H.; Tohge, T.; Viehover, P.; Fernie, A.R.; Weisshar, B.; Stracke, R. Natural variation in flavonol accumulation in Arabidopsis is determined by the flavonol glucosyltransferase BGLU6. J. Exp. Bot. 2016, 67, 1505–1517. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, M.H.; Jaafar, H.Z.E.; Karimi, E.; Ghasemzadeh, A. Primary, secondary metabolites, photosynthetic capacity and antioxidant activity of the malaysian herb Kacip fatimah (Labisia Pumila Benth) exposed to potassium fertilization under greenhouse conditions. Int. J. Mol. Sci. 2012, 13, 15321–15342. [Google Scholar] [CrossRef]
- Barku, V.Y.A.; Boye, A.; Ayaba, S. Phytochemical screening and assessment of wound healing activity of the leaves of Anogeissus leiocarpus. Eur. J. Exp. Biol. 2013, 3, 18–25. [Google Scholar]
- Kim, D.O.; Jeong, S.W.; Lee, C.Y. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem. 2003, 81, 321–326. [Google Scholar] [CrossRef]
- Djeridane, A.; Yousfi, M.; Nadjemi, B.; Boutassouna, D.; Stocker, P.; Vidal, N. Antioxidant activity of some algerian medicinal plants extracts containing phenolic compounds. Food Chem. 2006, 97, 654–660. [Google Scholar] [CrossRef]
- Oliveira, M.D.; Cipolatti, E.P.; Furlong, E.B.; Soares, L.D.S. Phenolic compounds and antioxidant activity in fermented rice (Oryza sativa) bran. Ciência Tecnol. De Aliment. Camp. 2012, 32, 531–537. [Google Scholar] [CrossRef] [Green Version]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (frap) as a measure of ‘‘antioxidant power’’: The frap assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Sample Availability: Samples of the sabah snake grass are available from the authors. |


| Treatment | Herbal Yield (g) | Total Phenolic Content (mg GAE/DW) | Total Flavonoid Content (mg quercetin/g DW) |
|---|---|---|---|
| Harvesting age (HA) | |||
| Week 8 | 15.43 ± 1.51 c | 6.17 ± 0.17 c | 4.31 ± 0.31 c |
| Week 12 | 29.00 ± 1.79 b | 6.87 ± 0.11 b | 6.21 ± 0.33 b |
| Week 16 | 129.56 ± 2.41 a | 10.84 ± 0.24 a | 7.32 ± 0.40 a |
| Harvesting frequency (HF) | |||
| Harvest 1 | 17.07 ± 1.26 c | 10.3 ± 0.14 a | 7.13 ± 0.38 a |
| Harvest 2 | 61.08 ± 2.21 b | 7.75 ± 0.19 b | 6.02 ± 0.33 b |
| Harvest 3 | 96.04 ± 2.23 a | 5.82 ± 0.19 c | 4.69 ± 0.34 c |
| F test | |||
| Harvesting age | ** | ** | ** |
| Harvesting frequency | ** | ** | ** |
| HA × HF | ** | ** | ns |
| Treatment | DPPH Scavenging Activity (% Inhibition) | Ferric Reducing Power Assay (µmol Fe (II)/g DW) |
|---|---|---|
| Harvesting age (HA) | ||
| Week 8 | 70.40 ± 0.36 c | 467.41 ± 10.25 c |
| Week 12 | 72.31 ± 0.40 b | 497.64 ± 4.50 b |
| Week 16 | 74.04 ± 0.30 a | 518.88 ± 8.79 a |
| Harvesting frequency (HF) | ||
| Harvest 1 | 74.76 ± 0.38 a | 537.72 ± 8.79 a |
| Harvest 2 | 72.33 ± 0.34 b | 490.37 ± 6.44 b |
| Harvest 3 | 69.67 ± 0.34 c | 455.84 ± 8.31 c |
| F test | ||
| Harvesting age | ** | ** |
| Harvesting frequency | ** | ** |
| HA × HF | ns | * |
| Treatment | C-glycosyl Flavone (µg/g) | ||||
|---|---|---|---|---|---|
| Shaftoside | Iso-Orientin | Orientin | Iso-Vitexin | Vitexin | |
| Harvesting age (HA) | |||||
| Week 8 | 5.32 ± 0.08 a | 1.59 ± 0.05 a | 0.56 ± 0.31 a | 0.45 ± 0.36 a | 0.09 ± 0.26 a |
| Week 12 | 7.12 ± 0.55 a | 1.31 ± 0.96 a | 0.65 ± 0.11 a | 0.29 ± 0.22 a | 0.33 ± 0.52 a |
| Week 16 | 7.39 ± 0.91 a | 2.31 ± 0.68 a | 0.66 ± 0.36 a | 0.65 ± 0.82 a | 0.32 ± 0.81 a |
| Harvesting frequency (HF) | |||||
| Harvest 1 | 11.27 ± 0.91 a | 3.07 ± 0.84 a | 1.09 ± 0.28 a | 0.83 ± 0.88 a | nd |
| Harvest 2 | 5.37 ± 0.29 b | 0.78 ± 0.31 b | 0.56 ± 0.41 ab | 0.21 ± 0.14 a | 0.51 ± 0.89 a |
| Harvest 3 | 3.20 ± 0.94 b | 0.49 ± 0.10 b | 0.23 ± 0.08 b | 0.36 ± 0.37 a | 0.23 ± 0.26 ab |
| F test | |||||
| Harvesting age | ns | ns | ns | ns | ns |
| Harvesting frequency | ** | * | ** | ns | ns |
| HA × HF | ns | ns | ns | ns | ns |
| Variables | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Yield | 1 | |||||||||
| 2 | TPC | 0.94 ** | 1 | ||||||||
| 3 | TFC | 0.90 ** | 0.74 * | 1 | |||||||
| 4 | FRAP | 0.83 * | 0.82 * | 0.88 * | 1 | ||||||
| 5 | DPPH | 0.91 ** | 0.97 ** | 0.81 * | 0.89 * | 1 | |||||
| 6 | Shaftoside | 0.36 | 0.38 | 0.20 | 0.13 | 0.26 | 1 | ||||
| 7 | Iso-orientin | 0.37 | 0.32 | 0.45 | 0.44 | 0.23 | 0.09 | 1 | |||
| 8 | Orientin | 0.25 | 0.25 | 0.28 | 0.48 | 0.27 | 0.28 | 0.03 | 1 | ||
| 9 | Iso-vitexin | 0.21 | 0.29 | 0.06 | 0.09 | 0.21 | 0.01 | 0.12 | 0.49 | 1 | |
| 10 | Vitexin | 0.41 | 0.32 | 0.31 | 0.47 | 0.40 | 0.22 | 0.29 | 0.36 | 0.38 | 1 |
| Variables | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Yield | 1 | |||||||||
| 2 | TPC | −0.99 ** | 1 | ||||||||
| 3 | TFC | −0.89 ** | 0.89 * | 1 | |||||||
| 4 | FRAP | −0.93 ** | 0.97 ** | 0.90 * | 1 | ||||||
| 5 | DPPH | −0.98 ** | 0.99 ** | 0.91 * | 0.97 ** | 1 | |||||
| 6 | Shaftoside | −0.95 ** | 0.92 * | 0.69 * | 0.82 * | 0.91 * | 1 | ||||
| 7 | Iso-orientin | −0.84 * | 0.85 * | 0.60 | 0.84 * | 0.84 * | 0.86 * | 1 | |||
| 8 | Orientin | −0.91 ** | 0.92 * | 0.74 * | 0.91 * | 0.90 * | 0.90 * | 0.88 * | 1 | ||
| 9 | Iso-vitexin | −0.43 | 0.42 | 0.40 | 0.42 | 0.42 | 0.25 | 0.15 | 0.19 | 1 | |
| 10 | Vitexin | −0.35 | −0.37 | −0.14 | −0.46 | −0.32 | −0.26 | −0.46 | −0.40 | −0.27 | 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd Samat, N.M.A.; Ahmad, S.; Awang, Y.; Bakar, R.A.H.; Hakiman, M. Alterations in Herbage Yield, Antioxidant Activities, Phytochemical Contents, and Bioactive Compounds of Sabah Snake Grass (Clinacanthus Nutans L.) with Regards to Harvesting Age and Harvesting Frequency. Molecules 2020, 25, 2833. https://doi.org/10.3390/molecules25122833
Abd Samat NMA, Ahmad S, Awang Y, Bakar RAH, Hakiman M. Alterations in Herbage Yield, Antioxidant Activities, Phytochemical Contents, and Bioactive Compounds of Sabah Snake Grass (Clinacanthus Nutans L.) with Regards to Harvesting Age and Harvesting Frequency. Molecules. 2020; 25(12):2833. https://doi.org/10.3390/molecules25122833
Chicago/Turabian StyleAbd Samat, Nur Mardhiati Afifa, Syahida Ahmad, Yahya Awang, Ros Azrinawati Hana Bakar, and Mansor Hakiman. 2020. "Alterations in Herbage Yield, Antioxidant Activities, Phytochemical Contents, and Bioactive Compounds of Sabah Snake Grass (Clinacanthus Nutans L.) with Regards to Harvesting Age and Harvesting Frequency" Molecules 25, no. 12: 2833. https://doi.org/10.3390/molecules25122833
APA StyleAbd Samat, N. M. A., Ahmad, S., Awang, Y., Bakar, R. A. H., & Hakiman, M. (2020). Alterations in Herbage Yield, Antioxidant Activities, Phytochemical Contents, and Bioactive Compounds of Sabah Snake Grass (Clinacanthus Nutans L.) with Regards to Harvesting Age and Harvesting Frequency. Molecules, 25(12), 2833. https://doi.org/10.3390/molecules25122833