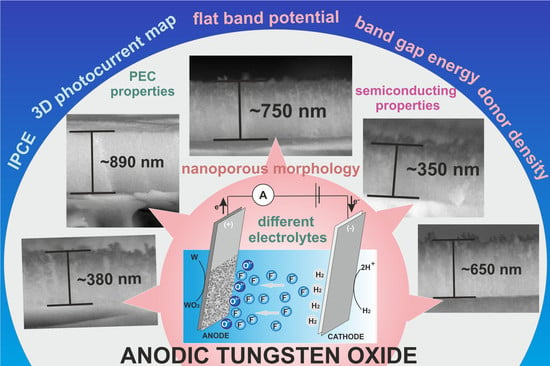
Improving Photoelectrochemical Properties of Anodic WO3 Layers by Optimizing Electrosynthesis Conditions
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Preparation of Anodic WO3 Layers
4.2. Characterization of Anodic WO3 Layers
4.3. Electrochemical and Photoelectrochemical Measurements
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- White, C.M.; Gillaspie, D.T.; Whitney, E.; Lee, S.-H.; Dillon, A.C. Flexible electrochromic devices based on crystalline WO3 nanostructures produced with hot-wire chemical vapor deposition. Thin Solid Films 2009, 517, 3596–3599. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, C.; Mei, Z.; Liu, X.; Qu, X.; Li, Y.; Li, S.; Qi, W.; Zhang, Y.; Ye, J.; et al. Monoclinic tungsten oxide with {100} facet orientation and tuned electronic band structure for enhanced photocatalytic oxidations. ACS Appl. Mater. Interfaces 2016, 8, 10367–10374. [Google Scholar] [CrossRef] [PubMed]
- Zhan, F.; Li, J.; Li, W.; Liu, Y.; Xis, R.; Yang, Y.; Li, Y.; Chen, Q. In situ formation of CuWO4/WO3 heterounction plates array films with enhanced photoelectrochemical propertie. Int. J. Hydrogen Energy 2015, 40, 6512–6520. [Google Scholar] [CrossRef]
- Breedon, M.; Spizzirri, P.; Taylor, M.; du Plessis, J.; McCulloch, D.; Zhu, J.; Yu, L.; Hu, Z.; Rix, C.; Wlodarski, W.; et al. Synthesis of nanostructured tungsten oxide thin films: A simple, controllable, inexpensive sol-gel method. Cryst. Growth Des. 2010, 10, 430–439. [Google Scholar] [CrossRef]
- Corby, S.; Francas, L.; Selim, S.; Sachs, M.; Blackman, C.; Kafizas, A.; Durrant, J.R. Water oxidation and electron extraction kinetics in nanostructured tungsten trioxide phooanodes. J. Am. Chem. Soc. 2018, 140, 16168–16177. [Google Scholar] [CrossRef] [PubMed]
- Ou, J.Z.; Rani, R.A.; Balendhran, S.; Zoolfakar, A.S.; Field, M.R.; Zhuiykov, S.; O’Mullane, A.P.; Kalantar-zadeh, K. Anodic formation of a thick three-dimensional nanoporous WO3 film and its photocatalytic property. Electrochem. Commun. 2013, 27, 128–132. [Google Scholar] [CrossRef]
- Li, L.; Zhao, X.; Pan, D.; Li, G. Nanotube array-like WO3/W photoanode fabricated by electrochemical anodization for photoelectrocatalytic overall water splitting. Chin. J. Catal. 2017, 38, 2132–2140. [Google Scholar] [CrossRef]
- Syrek, K.; Zaraska, L.; Zych, M.; Sulka, G.D. The effect of anodization conditions on the morphology of porous tungsten oxide layers formed in aqueous solution. J. Electroanal. Chem. 2018, 829, 106–115. [Google Scholar] [CrossRef]
- Rahmani, M.B.; Yaacob, M.H.; Sabri, Y.M. Hydrogen sensors based on 2D WO3 nanosheets prepared by anodization. Sensor Actut. B−Chem. 2017, 251, 57–64. [Google Scholar] [CrossRef]
- Lai, C.W. Photocatalysis and photoelectrochemical properties of tungsten trioxide nanostructured films. Sci. World J. 2014, 843587. [Google Scholar] [CrossRef]
- de Tacconi, N.R.; Chenthamarakshan, C.R.; Yogeeswaran, G.; Watcharenwong, A.; de Zoysa, R.S.; Basit, N.A.; Rajeshwar, K. Nanoporous TiO2 and WO3 films by anodization of titanium and tungsten substrates: Influence of process variables on morphology and photoelectrochemical response. J. Phys. Chem. B 2006, 110, 25346–25355. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Gil, K.R.; Wiggenhorn, C.; Brunschwig, B.S.; Lewis, N.S. Comparison between the quantum yields of compact and porous WO3 photoanodes. J. Phys. Chem. C 2013, 117, 14947–14957. [Google Scholar] [CrossRef] [Green Version]
- Tsuchiya, H.; Macak, J.M.; Sieber, I.; Taveira, L.; Ghicov, A.; Sirotna, K.; Schmuki, P. Self-organized porous WO3 formed in NaF electrolytes. Electrochem. Commun. 2005, 7, 295–298. [Google Scholar] [CrossRef]
- Watcharenwong, A.; Chanmanee, W.; de Tacconi, N.R.; Chenthamarakshan, C.R.; Kajitvichyanukul, P.; Rajeshwar, K. Anodic growth of nanoporous WO3 films: Morphology, photoelectrochemical response and photocatalytic activity for methylene blue and hexavalent chrome conversion. J. Electroanal. Chem. 2008, 612, 112–120. [Google Scholar] [CrossRef]
- Ahmadi, E.; Ng, C.Y.; Razak, K.A.; Lockman, Z. Preparation of anodic nanoporous WO3 film using oxalic acid as electrolyte. J. Alloy. Compd. 2017, 704, 518–527. [Google Scholar] [CrossRef]
- Lai, C.W.; Hamid, S.B.A.; Sreekantan, S. A novel solar driven photocatalyst: Well-aligned anodic WO3 nanotubes. Int. J. Photoenergy 2013, 745301. [Google Scholar] [CrossRef] [Green Version]
- Fernandez−Domene, R.M.; Sanchez-Tovar, R.; Lucas−Granados, B.; Rosello-Marquez, G.; Garcia−Anton, J. A simple method to fabricate high-performance nanostructured WO3 photocatalysts adjusted morphology in the presence of complexing agents. Mater. Des. 2017, 116, 160–170. [Google Scholar] [CrossRef]
- Sadek, A.Z.; Zheng, H.; Breedon, M.; Bansal, V.; Bhargava, S.K.; Latham, K.; Zhu, J.; Yu, L.; Hu, Z.; Spizzirri, P.G.; et al. High-temperature anodized WO3 nanoplatelet films for photosensitive devices. Langmuir 2009, 25, 9545–9551. [Google Scholar] [CrossRef]
- Altomare, M.; Pfoch, O.; Tighineanu, A.; Kirchgeorg, R.; Lee, K.; Selli, E.; Schmuki, P. Molten o-H3PO4: A new electrolyte for the anodic synthesis of self-organized oxide structures-WO3 nanochannel layers and others. J. Am. Chem. Soc. 2015, 137, 5646–5649. [Google Scholar] [CrossRef] [Green Version]
- Lai, C.W. WO3 nanoplates film: Formation and photocatalytic oxidation studies. J. Nanomater. 2015, 63587. [Google Scholar] [CrossRef]
- Chen, W.-H.; Lai, M.-Y.; Tsai, K.-T.; Liu, C.-Y.; Wang, Y.-L. Spontaneous formation of ordered nanobubbles in anodic tungsten oxide during anodization. J. Phys. Chem. C 2011, 115, 18406–18411. [Google Scholar] [CrossRef]
- Qin, L.; Chen, Q.; Lan, R.; Jiang, R.; Quan, X.; Xu, B.; Zhang, F.; Jia, Y. Effect of anodization parameters on morphology and photocatalysis properties of TiO2 nanotube arrays. J. Mater. Sci. Technol. 2015, 31, 1059–1064. [Google Scholar] [CrossRef]
- Zhu, T.; Chong, M.N.; Chan, E.S. Nanostructured tungsten trioxide thin films synthesized for photoelectrocatalytic water oxidation: A review. ChemSusChem 2014, 7, 2974–2997. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Domene, R.M.; Sanchez-Tovar, R.; Lucas-Granados, B.; Garcia-Anton, J. Improvement in photocatalytic activity of stable WO3 nanoplatelet globular clusters arranged in a tree-like fashion: Influence of rotation velocity during anodization. App. Catal. B−Environ. 2016, 189, 266–282. [Google Scholar] [CrossRef] [Green Version]
- Caramori, S.; Cristino, V.; Meda, L.; Tacca, A.; Argazzi, R.; Bignozzi, C.A. Efficient anodically grown WO3 for photoelectrochemical water splitting. Energy Procedia 2012, 22, 127–136. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.; Kim, D.; Lee, K.; Roy, P.; Schmuki, P. Direct anodic growth of thick WO3 mesosponge layers and characterization of their photoelectrochemical response. Electrochim. Acta 2010, 56, 828–833. [Google Scholar] [CrossRef]
- Mohamed, A.M.; Shaban, S.A.; El Sayed, H.A.; Alanadouli, B.E.; Allam, N.K. Morphology-photoactivity relationship: WO3 nanostructured films for solar hydrogen production. Int. J. Hydrogen Energy 2016, 41, 866–872. [Google Scholar] [CrossRef]
- Chai, Y.; Tam, C.W.; Beh, K.P.; Yam, F.K.; Hassan, Z. Porous WO3 formed by anodization in oxalic acid. J. Porous Mater. 2013, 20, 997–1002. [Google Scholar] [CrossRef]
- Ou, J.Z.; Balendhran, S.; Field, M.R.; McCulloch, D.G.; Zoolfakar, A.S.; Rani, R.A.; Zhuiykov, S.; O’Mullane, A.P.; Kalantar-zadeh, K. The anodized crystalline WO3 nanoporous network with enhanced electrochromic properties. Nanoscale 2012, 4, 5980. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, X.; Deng, H. A comprehensive study on electrochemical polishing of tungsten. Appl. Surf. Sci. 2019, 475, 587–597. [Google Scholar] [CrossRef]
- Ghicov, A.; Schmuki, P. Self-ordering electrochemistry: A review on growth and functionality of TiO2 nanotubes and other self-aligned MOx structures. Chem. Commun. 2009, 2791–2808. [Google Scholar] [CrossRef] [PubMed]
- Sulka, G.D. Introduction to anodization of metals. In Nanostructured Anodic Metal Oxides, Synthesis and Applications; Sulka, G.D., Ed.; Matthew Deans: Amsterdam, The Netherlands, 2020; pp. 1–34. [Google Scholar]
- Sopha, H.; Macak, J.M. Recent advancements in the synthesis, properties and applications of anodic self-organized nanotube layers. In Nanostructured Anodic Metal Oxides: Synthesis and Applications; Sulka, G.D., Ed.; Matthew Deans: Amsterdam, The Netherlands, 2020; pp. 173–210. [Google Scholar]
- Syrek, K.; Zych, M.; Zaraska, L.; Sulka, G.D. Influence of annealing conditions on anodic tungsten oxide layers and their photoelectrochemical activity. Electrochim. Acta 2017, 231, 61–68. [Google Scholar] [CrossRef]
- Syrek, K.; Sennik−Kubiec, A.; Rodriguez−Lopez, J.; Rutkowska, M.; Żmudzki, P.; Hnida-Gut, K.E.; Grudzień, J.; Chmielarz, L.; Sulka, G.D. Reactive and morphological trends on porous anodic TiO2 substrates obtained at different annealing temperatures. Int. J. Hydrog. Energy 2020, 45, 4376–4389. [Google Scholar] [CrossRef]
- Yan, J.; Wang, T.; Wu, G.; Dai, W.; Guan, N.; Li, L.; Gong, J. Tungsten oxide single crystal nanosheets for enhanced multichannel solar light harvesting. Adv. Mater. 2015, 27, 1580–1586. [Google Scholar] [CrossRef]
- Nakajima, T.; Hagino, A.; Nakamura, T.; Tsuchiya, T.; Sayama, K. WO3 nanosponge photoanodes with high applied bias photon-to-current efficiency for solar hydrogen and peroxydisulfate production. J. Mater. Chem. A 2016, 4, 17809–17818. [Google Scholar] [CrossRef] [Green Version]
- Bignozzi, C.A.; Caramori, S.; Cristino, V.; Argazzi, R.; Meda, L.; Tacca, A. Nanostructured photoelectrodes based on WO3: Applications to photooxidation of aqueous electrolytes. Chem. Soc. Rev. 2013, 42, 2228–2246. [Google Scholar] [CrossRef]
- Bolts, J.M.; Wrighton, M.S. Correlation of photocurrent-voltage curves with flat-band potential for stable photoelectrodes for photoelectrolysis of water. J. Phys. Chem. 1976, 80, 2641–2645. [Google Scholar] [CrossRef]
- Wysocka, I.; Kowalska, E.; Trzciński, K.; Łapiński, M.; Nowaczyk, G.; Zielińska−Jurek, A. UV-Vis-induced degradation of phenol over magnetic photocatalysts modified with Pt, Pd, Cu and Au nanoparticles. Nanomaterials 2018, 8, 28. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Domene, R.M.; Sanchez-Tovar, R.; Lucas-Granados, B.; Munoz-Portero, M.J.; Garcia-Anton, J. Elimination of pesticide atrazine by photoelectrocatalysis using a photoanode based on WO3 nanosheets. Chem. Eng. J. 2018, 350, 1114–1124. [Google Scholar] [CrossRef]
- Su, L.; Zhang, L.; Fang, J.; Xu, M.; Lu, Z. Electrochromic and photoelectrochemical behawior of electrodeposited tungsten trioxide films. Sol. Energy Mater. Sol. Cells 1999, 58, 133–140. [Google Scholar] [CrossRef]
- Wang, G.; Ling, Y.; Wang, H.; Yang, X.; Wang, C.; Zhang, J.Z.; Li, Y. Hydrogen-treated WO3 nanoflakes show enhanced photostability. Energy Environ. Sci. 2012, 5, 6180–6187. [Google Scholar] [CrossRef]
- Yagi, M.; Maruyama, S.; Sone, K.; Nagai, K.; Norimatsu, T. Preparation and photoelectrocatalytic activity of a nano-structured WO3 platelet film. J. Solid State Chem. 2008, 181, 175–182. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Li, W.; Han, S.; Liu, C. Photoelectrochemical properties and photocatalytic activity of nitrogen-doped nanoporous WO3 photoelectrodes under visible light. Appl. Surf. Sci. 2012, 258, 5038–5045. [Google Scholar] [CrossRef]
- Zhang, R.; Ning, F.; Xu, S.; Zhou, L.; Shao, M.; Wei, M. Oxygen vacancy engineering of WO3 toward largely enhanced photoelectrochemical water splitting. Electrochim. Acta 2018, 274, 217–223. [Google Scholar] [CrossRef]
- Ou, J.Z.; Ahmad, M.Z.; Latham, K.; Kalantar−zadeh, K.; Sberveglieri, G.; Wlodarski, W. Synthesis of the nanostructured WO3 via anodization at elevated temperature for H2 sensing applications. Procedia Eng. 2011, 25, 247–251. [Google Scholar] [CrossRef] [Green Version]
- Syrek, K.; Kapusta−Kołodziej, J.; Jarosz, M.; Sulka, G.D. Effect of electrolyte agitation on anodic titanium dioxide (ATO) growth and its photoelectrochemical properties. Electrochim. Acta 2015, 180, 801–810. [Google Scholar] [CrossRef]
- Zhu, T.; Chong, M.N.; Phuan, Y.W.; Chan, E.-S. Electrochemically synthesized tungsten trioxide nanostructures for photoelectrochemical water splitting: Influence of heat treatment on physicochemical properties, photocurrent densities and electron shuttling. Colloids Surf. Physicochem. Eng. Asp. 2015, 484, 297–303. [Google Scholar] [CrossRef]
- Zhuang, H.; Sun, L.; Chen, Z.; Lin, C. Self-organized TiO2 nanotubes in mixed organic-inorganic electrolytes and their photoelectrochemical performance. Electrochim. Acta 2009, 54, 6536–6542. [Google Scholar]
- Liu, Z.; Zhang, Q.; Zhao, T.; Zhai, J.; Jiang, L. 3-D vertical arrays of TiO2 nanotubes on Ti meshes: Efficient photoanodes for water photoelectrolysis. J. Mater. Chem. 2011, 21, 10354–10358. [Google Scholar] [CrossRef]
- Kapusta-Kołodziej, J.; Chudecka, A.; Sulka, G.D. 3D nanoporous titania formed by anodization as a promising photoelectrode material. J. Electroanal. Chem. 2018, 823, 221–233. [Google Scholar] [CrossRef]
- Bard, A.J. Photoelectrochemistry. Science 1980, 207, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Horcas, I.; Fernandez, R.; Gomez−Rodriguez, J.M.; Colchero, J.; Gomez-Herrero, J.; Baro, A.M. WSMX: A software for scanning probe microscopy and a tool for nanotechnology. Rev. Sci. Instrum. 2007, 78, 0137705. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: WO3 samples are not available from the authors. |









| Electrolyte Composition; Time of Anodization; Applied Voltage | Morphology; Oxide Thickness | Current Density (at a Given Potential) | Electrolyte | Light Source and Intensity | Ref. |
|---|---|---|---|---|---|
| 0.15 M NH4F (glycerol/water 50/50 vol %); 1 h; 40 V | Nanotubes | 0.38 mA cm−2 (0.6 V vs. SCE) | 0.5 M Na2SO4, 25 vol % methanol | LED (15 mW cm−2) | [7] |
| 1 M HNO3; 1 h; 40 V | Nanoflakes | 1.17 mA cm−2 (1.2 V vs. SCE) | 1 M H2SO4 | Xe lamp (AM 1.5 G filter; 100 mW cm−2) | [27] |
| 10 wt% K2HPO4/glycerol; 20 h; 50 V | Mesoporous layers; 2.5 μm | ~1.4 mA cm−2 (1.0 V vs. Ag/AgCl) | 1 M HClO4 | Xe lamp (AM1.5 filter) | [26] |
| 0.1 M NaF; 24 h; 60 V | Porous film; 2. 59 μm | 0.75 mA cm−2 (1.23 V vs. RHE) | 0.1 M HCl | Xe lamp (100 mW cm−2) | [12] |
| 0.15 M NaF; 1 h; 60 V | Nanoporous | 3.21 mA cm−2 (2.0 V vs. Ag/AgCl) | 0.5 M Na2SO4 | Xe lamp | [14] |
| 0.15 M NaF; 1 h; 60 V | Nanoporous | 0.63 mA cm−2 (2.0 V vs. Ag/AgCl) | 0.5 M Na2SO4 | Xe lamp | [11] |
| Anodization Conditions | WO3 Sample Label | Photoelectrochemical Measurements | UV-Vis Diffuse Reflectance Spectroscopy Measurements |
|---|---|---|---|
| 1 M (NH4)2SO4 and 0.075 M NH4F; 50 V; 240 min | B | 2.69 ± 0.05 | 2.90 ± 0.06 |
| 1 M Na2SO4 and 0.12 M NaF; 40 V; 120 min | C | 2.71 ± 0.05 | 2.91 ± 0.06 |
| 1 M Na2SO4 and 0.19 M NH4F; 40 V; 15 min | D | 2.72 ± 0.05 | 2.87 ± 0.06 |
| 0.27 M NH4F in 2.2 wt.% H2O in ethylene glycol; 10 V; 60 min | G | 2.68 ± 0.05 | 3.00 ± 0.06 |
| 0.15 M NH4F; 30 V; 30 min | F | 2.74 ± 0.05 | 2.79 ± 0.06 |
| Anodization Conditions | WO3 Sample Label | Efb vs. SCE / V | Nd / cm−3 |
|---|---|---|---|
| 1 M (NH4)2SO4 and 0.075 M NH4F; 50 V; 240 min | B | −0.08 | (3.64 ± 0.22) × 1021 |
| 1 M Na2SO4 and 0.12 M NaF; 40 V; 120 min | C | −0.25 | (2.64 ± 0.29) × 1021 |
| 1 M Na2SO4 and 0.19 M NH4F; 40 V; 15 min | D | −0.25 | (1.53 ± 0.17) × 1021 |
| 0.27 M NH4F in 2.2 wt.% H2O in ethylene glycol; 10 V; 60 min | G | −0.20 | (3.08 ± 0.45) × 1021 |
| 0.15 M NH4F; 30 V; 30 min | F | −0.24 | (1.18 ± 0.35) × 1021 |
| Electrolyte Composition | Time of Anodization / min | Applied Voltage / V | WO3 Sample Label |
|---|---|---|---|
| 1 M (NH4)2SO4 + 0.075 M NH4F | 240 | 50 | B |
| 1 M Na2SO4 + 0.12 M NaF | 120 | 40 | C |
| 1 M Na2SO4 + 0.19 M NH4F | 15 | 40 | D |
| 0.27 M NH4F + 2.2 wt.% H2O, ethylene glycol based solution | 60 | 10 | G |
| 0.15 M NH4F | 30 | 30 | F |
| 1.8 M NaOH | 45 s | 35 | Z |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zych, M.; Syrek, K.; Zaraska, L.; Sulka, G.D. Improving Photoelectrochemical Properties of Anodic WO3 Layers by Optimizing Electrosynthesis Conditions. Molecules 2020, 25, 2916. https://doi.org/10.3390/molecules25122916
Zych M, Syrek K, Zaraska L, Sulka GD. Improving Photoelectrochemical Properties of Anodic WO3 Layers by Optimizing Electrosynthesis Conditions. Molecules. 2020; 25(12):2916. https://doi.org/10.3390/molecules25122916
Chicago/Turabian StyleZych, Marta, Karolina Syrek, Leszek Zaraska, and Grzegorz D. Sulka. 2020. "Improving Photoelectrochemical Properties of Anodic WO3 Layers by Optimizing Electrosynthesis Conditions" Molecules 25, no. 12: 2916. https://doi.org/10.3390/molecules25122916
APA StyleZych, M., Syrek, K., Zaraska, L., & Sulka, G. D. (2020). Improving Photoelectrochemical Properties of Anodic WO3 Layers by Optimizing Electrosynthesis Conditions. Molecules, 25(12), 2916. https://doi.org/10.3390/molecules25122916