Effect of Naringenin and Its Derivatives on the Probing Behavior of Myzus persicae (Sulz.)
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
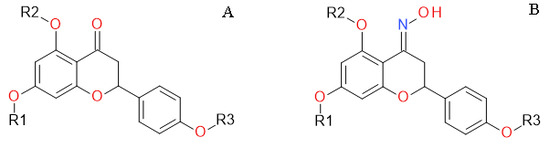
4.1. Naringenin and Naringenin Derivatives
4.2. Aphid and Plant Cultures
4.3. Preparation and Application of Compounds
4.4. Aphid Probing Behavior
4.5. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Venkateswara, R.P.; SDVS, K.; Rohini, P.; Bhagyasree, P. Flavonoid: A review on naringenin. J. Pharmacogn. Phytochem. 2017, 6, 2778–2783. [Google Scholar]
- Khan, M.K.; Zill-E-Huma, O.; Dangles, O. A comprehensive review on flavanones, the major citrus polyphenols. J. Food Compos. 2014, 33, 85–104. [Google Scholar] [CrossRef]
- Patton, C.A.; Ranney, T.G.; Burton, J.D.; Walgenbach, J.F. Feeding responses of japanese beetle to naturally occurring metabolites found in rosaceous plants. J. Environ. Hort. 1997, 15, 222–227. [Google Scholar]
- Su, Q.; Zhou, Z.; Zhang, J.; Shi, C.; Zhang, G.; Jin, Z.; Wang, W.; Li, C. Effect of plant secondary metabolites on common cutworm, Spodoptera litura (Lepidoptera: Noctuidae). Entomol. Res. 2017, 48, 18–26. [Google Scholar] [CrossRef]
- Riddick, E.W.; Wu, Z.; Eller, F.J.; Berhow, M.A. Do bioflavonoids in Juniperus virginiana heartwood stimulate oviposition in the ladybird Coleomegilla maculata? Int. J. Insect Sci. 2018, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, I.G.; Palottini, F.; Macri, I.; Galmarini, C.R.; Farina, W.M. Appetitive behavior of the honeybee Apis mellifera in response to phenolic compounds naturally found in nectars. J. Exp. Biol. 2019, 222, 189910. [Google Scholar] [CrossRef] [Green Version]
- Wellings, P.W.; Ward, S.A.; Dixon, A.F.G.; Rabbinge, R. Crop Loss Assessment. In Aphids, Their Biology, Natural Enemies and Control; Minks, A.K., Harrewijn, P., Eds.; Elsevier: Amsterdam, The Netherlands, 1989; Volume C, pp. 49–64. [Google Scholar]
- Blackman, R.L.; Eastop, V.F. Aphids on the World’s Crops: An Identification Guide; John Wiley and Sons: Chichester, UK; New York, NY, USA; Brisbane, Australia; Toronto, Japan; Singapore, 1985. [Google Scholar]
- Goławska, S.; Sprawka, I.; Łukasik, I.; Goławski, A. Are naringenin and quercetin useful chemicals in pest-management strategies? J. Pest. Sci. 2014, 87, 173–180. [Google Scholar] [CrossRef] [Green Version]
- Dedryver, C.A.; Le Ralec, A.; Fabre, F. The conflicting relationships between aphids and men: A review of aphid damage and control strategies. C. R. Biol. 2010, 333, 539–553. [Google Scholar] [CrossRef]
- Bass, C.; Puinean, A.M.; Zimmer, C.T.; Denholm, I.; Field, L.M.; Foster, S.P.; Gutbrod, O.; Nauen, R.; Slater, R.; Williamson, M.S. The evolution of insecticide resistance in the peach potato aphid, Myzus persicae. Insect Biochem. Mol. Biol. 2014, 51, 41–51. [Google Scholar] [CrossRef] [Green Version]
- Blake, R. Eu Neonicotinoid Ban Removes Vital Tools in Global Fight Against Pests. Outlooks Pest Manag. 2018, 29, 197–200. [Google Scholar] [CrossRef]
- Pickett, J.A. Lower terpenoids as natural insect control agents. In Ecological Chemistry and Biochemistry of Plant Terpenoids; Harborne, J.B., Tomas-Barberan, F.A., Eds.; Clarendon Press: Oxford, UK, 1991; pp. 297–313. [Google Scholar]
- Dayan, F.E.; Cantrell, C.l.; Duke, S.O. Natural products in crop protection. Bioorg. Med. Chem. 2009, 17, 4022–4034. [Google Scholar] [CrossRef] [PubMed]
- Lattanzio, V. Phenolic compounds: Introduction. In Natural Products; Ramawat, K.G., Me’rillon, J.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1543–1580. [Google Scholar]
- Powell, G.; Hardie, J.; Pickett, J. Effects of the antifeedant polygodial on plant penetration by aphids, assessed by video and electrical recording. Entomol. Exp. Appl. 1993, 68, 193–200. [Google Scholar] [CrossRef]
- Wróblewska-Kurdyk, A.; Gniłka, R.; Dancewicz, K.; Grudniewska, A.; Wawrzeńczyk, C.; Gabryś, B. β-thujone and its derivatives modify the probing behavior of the peach potato aphid. Molecules 2019, 24, 1847. [Google Scholar] [CrossRef] [Green Version]
- Wróblewska-Kurdyk, A.; Dancewicz, K.; Gliszczyńska, A.; Gabryś, B. New insight into the behaviour modifying activity of two natural sesquiterpenoids farnesol and nerolidol towards Myzus persicae (Sulzer) (Homoptera: Aphididae). Bull. Entomol. Res. 2020, 110, 249–258. [Google Scholar] [CrossRef]
- Gabryś, B.; Dancewicz, K.; Gliszczyńska, A.; Kordan, B.; Wawrzeńczyk, C. Systemic deterrence of aphid probing and feeding by β-damascone analogues. J. Pest. Sci. 2015, 88, 507–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stompor, M.; Dancewicz, K.; Gabryś, B.; Anioł, M. Insect antifeedant potential of xanthohumol, isoxanthohumol, and their derivatives. J. Agric. Food Chem. 2015, 63, 6749–6756. [Google Scholar] [CrossRef]
- Shelton, A.M.; Badenes-Perez, F.R. Concepts and applications of trap cropping in pest management. Annu. Rev. Entomol. 2006, 51, 285–308. [Google Scholar] [CrossRef] [Green Version]
- Fereres, A. Barrier crops as a cultural control measure of non-persistently transmitted aphid-borne viruses. Virus Res. 2000, 71, 221–231. [Google Scholar] [CrossRef]
- Cook, S.M.; Khan, Z.R.; Pickett, J.A. The use of push-pull strategies in integrated pest management. Annu. Rev. Entomol. 2007, 52, 375–400. [Google Scholar] [CrossRef] [Green Version]
- Grudniewska, A.; Dancewicz, K.; Białońska, A.; Ciunik, Z.; Gabryś, B.; Wawrzeńczyk, C. Synthesis of piperitone-derived halogenated lactones and their effect on aphid probing, feeding, and settling behavior. RSC Adv. 2011, 1, 498–510. [Google Scholar] [CrossRef]
- Paprocka, M.; Gliszczyńska, A.; Dancewicz, K.; Gabryś, B. Novel hydroxy- and epoxy-cis-jasmone and dihydrojasmone derivatives affect the foraging of the peach potato aphid Myzus persicae (Sulzer) (Homoptera: Aphididae). Molecules 2018, 23, 2362. [Google Scholar] [CrossRef] [PubMed]
- Dancewicz, K.; Gabryś, B.; Dams, I.; Wawrzeńczyk, C. Enantiospecific effect of pulegone and pulegone−derived lactones on settling and feeding of Myzus persicae (Sulz.). J. Chem. Ecol. 2008, 34, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Dancewicz, K.; Sznajder, K.; Załuski, D., Kordan; Gabryś, B. Behavioral sensitivity of Myzus persicae to volatile isporenoids in plant tissues. Entomol. Exp. Appl. 2016, 160, 229–240. [Google Scholar] [CrossRef]
- Gabryś, B.; Pawluk, M. Acceptability of different species of Brassicaceae as hosts for the cabbage aphid. Entomol. Exp. Appl. 1999, 91, 105–109. [Google Scholar] [CrossRef]
- Grudniewska, A.; Dancewicz, K.; Białońska, A.; Wawrzeńczyk, C.; Gabryś, B. Piperitone-derived saturated lactones: Synthesis and aphid behavior-modifying activity. J. Agric. Food Chem. 2013, 61, 3364–3372. [Google Scholar] [CrossRef]
- Tjallingii, W.F. Regulation of phloem sap feeding by aphids. In Regulatory Mechanisms in Insect Feeding; Chapman, R.F., de Boer, G., Eds.; Chapman & Hall: New York, NY, USA, 1995; pp. 190–209. [Google Scholar]
- Mayoral, A.M.; Tjallingii, W.F.; Castanera, P. Probing behavior of Diuraphis noxia on five cereal species with different hydroxyamic acid levels. Entomol. Exp. Appl. 1996, 78, 341–348. [Google Scholar] [CrossRef]
- Philippi, J.; Schliephake, E.; Jurgens, H.U.; Jansen, G.; Ordon, F. Feeding behavior of aphids on narrow-leafed lupin (Lupinus angustifolius) genotypes varying in the content of quinolizidine alkaloids. Entomol. Exp. Appl. 2005, 156, 37–51. [Google Scholar] [CrossRef]
- Schwarzkopf, A.; Rosenberger, D.; Niebergall, M.; Gershenzon, J.; Kunert, G. To feed or not to feed: Plant factors located in the epidermis, mesophyll, and sieve elements influence pea aphid’s ability to feed on legume species. PLoS ONE 2013, 8, e75298. [Google Scholar] [CrossRef] [Green Version]
- Ververidis, F.; Trantas, E.; Douglas, C.; Vollmer, G.; Kretzschmar, G.; Panopoulos, N. Biotechnology of flavonoids and other phenylpropanoid-derived natural products. Part I: Chemical diversity, impacts on plant biology and human health. Biotechnol. J. 2007, 2, 1214–1234. [Google Scholar] [CrossRef]
- Prado, E.; Tjallingii, W.F. Aphid activities during sieve element punctures. Entomol. Exp. Appl. 1994, 72, 157–165. [Google Scholar] [CrossRef]
- Powell, G.; Pirone, T.; Hardie, J. Aphid stylet activities during potyvirus acquisition from plants and in vitro system that correlate with subsequent transmission. Eur. J. Plant Pathol. 1995, 101, 411–420. [Google Scholar] [CrossRef]
- Martin, B.; Collar, J.L.; Tjallingii, W.F.; Fereres, A. Intracellular ingestion and salivation by aphids may cause the acquisition and inoculation of non-persistently transmitted plant viruses. J. Gen. Virol. 1997, 78, 2701–2705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brault, V.; Uzest, M.; Monsion, B.; Jacquot, E.; Blanc, S. Aphids as transport devices for plant viruses. C. R. Biol. 2010, 333, 524–538. [Google Scholar] [CrossRef]
- Kozłowska, J.; Potaniec, B.; Żarowska, B.; Anioł, M. Synthesis and biological activity of novel O-alkyl derivatives of naringenin and their oximes. Molecules 2017, 22, 1485. [Google Scholar] [CrossRef] [Green Version]
- Polonsky, J.; Bhatnagar, S.C.; Griffiths, D.C.; Pickett, J.A.; Woodcock, C.M. Activity of qassinoids as antifeedants against aphids. J. Chem. Ecol. 1989, 15, 933–998. [Google Scholar] [CrossRef] [PubMed]
- Halarewicz-Pacan, A.; Gabryś, B.; Dancewicz, K.; Wawrzeńczyk, C. Enantiospecific effect of limonene and limonene-derived bicyclic lactones on settling and probing behaviour of the peach-potato aphid Myzus persicae (Sulz.). J. Plant Prot. Res. 2003, 43, 133–142. [Google Scholar]
- Pettersson, J.; Tjallingii, W.F.; Hardie, J. Host-plant selection and feeding. In Aphids as Crop Pests; van Emden, H.F., Harrington, R., Eds.; CABI: Wallingford, UK, 2017; pp. 173–195. [Google Scholar]
- Tjallingii, W.F.; Hogen Esch, T.H. Fine-structure of aphid stylet routes in plant-tissues in correlation with EPG signals. Physiol. Entomol. 1993, 18, 317–328. [Google Scholar] [CrossRef]
Sample Availability: Not available. |


| Compound/EPG Parameter | Sample Size | Total Duration of Non-Probing (h) | Total Duration of Probing in Non-Phloem Tissues C + F + G (h) | Total Duration of Phloem Phase E1 + E2 (h) | Total Duration of Sap Ingestion Phase E2 (h) | Number of Probes (#) | Mean Duration of a Probe (h) |
|---|---|---|---|---|---|---|---|
| Control | n = 16 | 0.9 ± 1.2 A | 2.7 ± 1.3 A | 4.4 ± 1.7 A | 4.2 ± 1.8 A | 19.8 ± 10.0 A | 0.6 ± 0.6 A |
| 1 | n = 14 | 0.8 ± 0.5 aA | 2.7 ± 1.8 aA | 4.5 ± 2.1 aA | 4.5 ± 2.1 aA | 19.1 ± 13.7 aA | 0.8 ± 1.0 aA |
| 2 | n = 13 | 1.3 ± 1.1 aA | 4.0 ± 1.7 aB | 2.7 ± 2.4 aB | 2.6 ± 2.4 aA | 24.2 ± 16.3 aA | 1.2 ± 2.2 aA |
| 3 | n = 13 | 0.9 ± 1.6 aA | 3.2 ± 1.4 aA | 4.0 ± 1.8 aA | 3.9 ± 1.8 aA | 10.8 ± 8.4 aB | 1.5 ± 2.0 aB |
| 4 | n = 13 | 0.5 ± 0.5 aA | 3.5 ± 1.8 aA | 3.9 ± 2.1 aA | 3.9 ± 2.1 a A | 12.9 ± 6.9 aB | 0.8 ± 0.5 aB |
| 5 | n = 14 | 0.8 ± 0.8 aA | 3.3 ± 2.3 aA | 3.9 ± 2.8 aA | 3.9 ± 2.8 aA | 20.3 ± 15.1 aA | 0.8 ± 0.8 aA |
| 6 | n = 14 | 0.6 ± 0.7 aA | 2.3 ± 1.8 aA | 5.1 ± 2.3 aA | 5.1 ± 2.3 aA | 13.1 ± 10.3 aA | 1.5 ± 1.9 aA |
| 7 | n = 16 | 1.2 ± 1.2 aA | 3.5 ± 1.9 aA | 3.3 ± 2.1 aA | 3.3 ± 2.2 aA | 22.3 ± 12.3 aA | 0.6 ± 0.6 aA |
| 8 | n = 13 | 0.3 ± 0.2 aB | 2.9 ± 2.1 aA | 4.8 ± 2.2 aA | 4.7 ± 2.2 aA | 11.5 ± 7.8 aB | 1.3 ± 1.2 aB |
| 9 | n = 16 | 0.8 ± 0.6 aA | 2.8 ± 1.6 aA | 4.4 ± 1.9 aA | 4.4 ± 1.9 aA | 17.3 ± 12.3 aA | 0.8 ± 0.7 aA |
| 10 | n = 14 | 0.6 ± 0.6 a A | 3.7 ± 2.3 aA | 3.7 ± 2.7 aA | 3.7 ± 2.7 aA | 14.9 ± 11.7 aA | 1.4 ± 2.0 aA |
| 11 | n = 13 | 0.7 ± 0.6 aA | 3.7 ± 2.0 aA | 3.6 ± 2.2 aA | 3.5 ± 2.2 aA | 15.8 ± 7.0 aA | 0.8 ± 1.0 aA |
| 12 | n = 14 | 0.7 ± 0.4 aA | 4.3 ± 2.0 aB | 3.0 ± 2.2 aA | 3.0 ± 2.2 aA | 15.5 ± 10.5 aA | 0.7 ± 0.4 aA |
| 13 | n = 14 | 0.3 ± 0.3 a B | 2.1 ± 1.5 aA | 5.6 ± 1.7 aA | 5.6 ± 1.7 aB | 11.1 ± 8.8 a B | 1.1 ± 0.7 aB |
| 14 | n = 14 | 1.0 ± 0.7 aA | 2.9 ± 1.6 aA | 4.1 ± 2.1 aA | 4.1 ± 2.1 aA | 19.7 ± 9.4 aA | 0.5 ± 0.3 aA |
| 15 | n = 14 | 0.6 ± 0.8 aA | 2.0 ± 1.8 aA | 5.5 ± 2.4 aA | 5.5 ± 2.4 aA | 11.9 ± 12.1 aB | 2.0 ± 2.5 aB |
| 16 | n = 12 | 0.7 ± 0.9 aA | 2.3 ± 1.8 aA | 4.9 ± 2.2 aA | 4.9 ± 2.2 aA | 9.8 ± 7.8 aB | 2.2 ± 2.7 aB |
| 17 | n = 13 | 0.8 ± 0.7 aA | 2.8 ± 1.9 aA | 4.4 ± 2.5 aA | 4.4 ± 2.5 aA | 20.01 ± 4.2 aA | 0.7 ± 0.6 aA |
| Compound/EPG Parameter | Sample Size | Total Duration of Non-Probing (h) | Total Duration of Probing in Non-Phloem Tissues C + F + G (h) | Number of Probes | Time from 1st Probe to 1st Phloem Phase E1 (h) |
|---|---|---|---|---|---|
| Control | n = 16 | 0.4 ± 0.2 A | 1.6 ± 1.2 A | 12.7 ± 8.8 A | 2.0 ± 1.3 A |
| 1 | n = 13 | 0.7 ± 0.5 aA | 1.8 ± 1.4 aA | 15.8 ± 13.6 abA | 2.4 ± 1.8 abA |
| 2 | n = 13 | 0.7 ± 0.7 aA | 1.8 ± 1.1 aA | 10.5 ± 8.7 abA | 2.5 ± 1.5 abA |
| 3 | n = 12 | 0.2 ± 0.2 aB | 2.0 ± 1.2 aA | 4.4 ± 4.2 aB | 2.1 ± 1.2 abA |
| 4 | n = 13 | 0.2 ± 0.2 aA | 1.4 ± 1.2 aA | 4.9 ± 4.0 abB | 1.5 ± 1.3 abA |
| 5 | n = 13 | 0.3 ± 0.4 aA | 1.0 ± 0.7 aA | 8.6 ± 7.9 abA | 1.3 ± 1.0 abA |
| 6 | n = 14 | 0.4 ± 0.3 aA | 1.7 ± 1.5 aA | 8.7 ± 7.6 abA | 2.1 ± 1.8 abA |
| 7 | n = 15 | 0.7 ± 0.4 aB | 2.2 ± 1.2 aA | 16.7 ± 9.7 bA | 2.8 ± 1.4 bA |
| 8 | n = 13 | 0.2 ± 0.2 aB | 2.0 ± 1.6 aA | 6.8 ± 7.3 abB | 2.1 ± 1.7 abA |
| 9 | n = 16 | 0.4 ± 0.3 aA | 1.5 ± 0.8 aA | 8.4 ± 6.4 abA | 1.8 ± 0.9 abA |
| 10 | n = 13 | 0.2 ± 0.2 aA | 1.9 ± 1.7 aA | 6.2 ± 4.1 abA | 2.1 ± 1.8 abA |
| 11 | n = 12 | 0.4 ± 0.6 aA | 2.3 ± 2.4 aA | 8.3 ± 7.0 abA | 2.7 ± 2.6 abA |
| 12 | n = 14 | 0.4 ± 0.3 aA | 1.8 ± 1.6 aA | 9.2 ± 8.1 abA | 2.1 ± 1.9 abA |
| 13 | n = 14 | 0.2 ± 0.1 aB | 0.9 ± 0.6 aB | 5.4 ± 5.0 abB | 1.0 ± 0.8 aB |
| 14 | n = 12 | 0.5 ± 0.4 aA | 1.2 ± 0.8 aA | 11.2 ± 7.4 abA | 1.7 ± 1.1 abA |
| 15 | n = 14 | 0.2 ± 0.2 aA | 1.0 ± 0.8 aA | 5.2 ± 3.5 abB | 1.2 ± 0.9 abA |
| 16 | n = 12 | 0.5 ± 0.8 aA | 1.5 ± 1.5 aA | 5.2 ± 7.3 aB | 2.0 ± 2.0 abA |
| 17 | n = 11 | 0.4 ± 0.3 aA | 1.5 ± 0.7 aA | 10.9 ± 7.8 abA | 1.8 ± 1.0 abA |
| Compound/EPG Parameter | Sample Size * | Number of Phloem Phases E1 and E1 + E (#) | Number of Phloem Sap Ingestion Phases (#) | Number of Sustained Sap Ingestion Phases E2 > 10 min (#) | Sample Size ** | Mean Duration of 1st Phloem Phase E1 + E2 (h) | Mean Duration of Phloem Sap Ingestion Phase E2 (h) |
|---|---|---|---|---|---|---|---|
| Control | n = 16 | 4.4 ± 2.8 A | 4.4 ± 2.7 A | 2.6 ± 1.5 A | n = 16 | 1.7 ± 2.4 A | 2.1 ± 2.2 A |
| 1 | n = 14 | 1.9 ± 1.6 aB | 1.7 ± 1.2 aB | 1.4 ± 0.9 aA | n = 13 | 3.0 ± 2.3 abB | 3.4 ± 2.0 aB |
| 2 | n = 13 | 3.1 ± 1.7 aA | 3.1 ± 1.7 aA | 1.6 ± 1.1 aA | n = 13 | 1.1 ± 2.0 aA | 1.5 ± 2.0 aA |
| 3 | n = 13 | 2.4 ± 2.6 aB | 2.3 ± 2.3 aB | 1.5 ± 0.8 aA | n = 12 | 3.0 ± 2.4 abA | 3.2 ± 2.1 aA |
| 4 | n = 13 | 2.6 ± 1.3 aA | 2.6 ± 1.3 aA | 1.5 ± 1.2 aA | n = 13 | 1.9 ± 2.3 abA | 2.3 ± 2.0 aA |
| 5 | n = 14 | 3.1 ± 2.2 aA | 2.9 ± 2.1 aA | 1.8 ± 1.1 aA | n = 13 | 2.6 ± 3.1 abA | 2.8 ± 3.0 aA |
| 6 | n = 14 | 2.8 ± 1.8 aA | 2.7 ± 1.7 aA | 1.8 ± 1.3 aA | n = 14 | 2.7 ± 3.0 abA | 2.8 ± 2.7 aA |
| 7 | n = 16 | 1.9 ± 1.2 aB | 1.9 ± 1.2 aB | 1.6 ± 0.9 aA | n = 15 | 2.3 ± 2.2 abA | 2.5 ± 2.2 aA |
| 8 | n = 13 | 2.2 ± 1.6 aB | 2.2 ± 1.6 aB | 1.6 ± 0.8 aA | n = 13 | 3.7 ± 2.5 abB | 3.6 ± 2.4 aA |
| 9 | n = 16 | 2.9 ± 2.3 aA | 3.0 ± 2.4 aA | 1.6 ± 0.9 aA | n = 16 | 2.9 ± 2.5 abA | 3.0 ± 2.5 aA |
| 10 | n = 14 | 2.1 ± 1.6 aB | 2.1 ± 1.6 aB | 1.6 ± 1.2 aA | n = 13 | 3.4 ± 2.8 abB | 3.2 ± 2.8 aA |
| 11 | n = 13 | 2.9 ± 2.7 aA | 2.9 ± 2.7 aA | 2.0 ± 1.8 aA | n = 12 | 1.6 ± 2.2 abA | 2.1 ± 2.0 aA |
| 12 | n = 14 | 3.1 ± 1.8 aA | 2.8 ± 1.7 aA | 1.6 ± 0.8 aA | n = 14 | 2.7 ± 2.2 abA | 2.8 ± 2.2 aA |
| 13 | n = 14 | 3.1 ± 1.9 aA | 3.1 ± 1.9 aA | 2.0 ± 1.2 aA | n = 14 | 3.0 ± 3.1 abA | 3.3 ± 2.8 aA |
| 14 | n = 14 | 2.9 ± 2.2 aA | 2.8 ± 2.2 aA | 2.1 ± 1.5 aA | n = 12 | 2.0 ± 2.3 abA | 2.4 ± 2.0 aA |
| 15 | n = 14 | 1.8 ± 1.4 aB | 1.8 ± 1.4 aB | 1.4 ± 0.7 aB | n = 14 | 5.0 ± 2.7 bB | 4.7 ± 3.0 aB |
| 16 | n = 12 | 2.6 ± 1.8 aA | 2.5 ± 1.8 aA | 1.9 ± 1.7 aA | n = 12 | 3.6 ± 2.9 abB | 3.4 ± 2.9 aA |
| 17 | n = 13 | 1.7 ± 1.6 aB | 1.7 ± 1.6 aB | 1.5 ± 1.3 aA | n = 11 | 3.8 ± 2.7 abB | 4.0 ± 2.5 aB |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stec, K.; Kozłowska, J.; Wróblewska-Kurdyk, A.; Kordan, B.; Anioł, M.; Gabryś, B. Effect of Naringenin and Its Derivatives on the Probing Behavior of Myzus persicae (Sulz.). Molecules 2020, 25, 3185. https://doi.org/10.3390/molecules25143185
Stec K, Kozłowska J, Wróblewska-Kurdyk A, Kordan B, Anioł M, Gabryś B. Effect of Naringenin and Its Derivatives on the Probing Behavior of Myzus persicae (Sulz.). Molecules. 2020; 25(14):3185. https://doi.org/10.3390/molecules25143185
Chicago/Turabian StyleStec, Katarzyna, Joanna Kozłowska, Anna Wróblewska-Kurdyk, Bożena Kordan, Mirosław Anioł, and Beata Gabryś. 2020. "Effect of Naringenin and Its Derivatives on the Probing Behavior of Myzus persicae (Sulz.)" Molecules 25, no. 14: 3185. https://doi.org/10.3390/molecules25143185
APA StyleStec, K., Kozłowska, J., Wróblewska-Kurdyk, A., Kordan, B., Anioł, M., & Gabryś, B. (2020). Effect of Naringenin and Its Derivatives on the Probing Behavior of Myzus persicae (Sulz.). Molecules, 25(14), 3185. https://doi.org/10.3390/molecules25143185