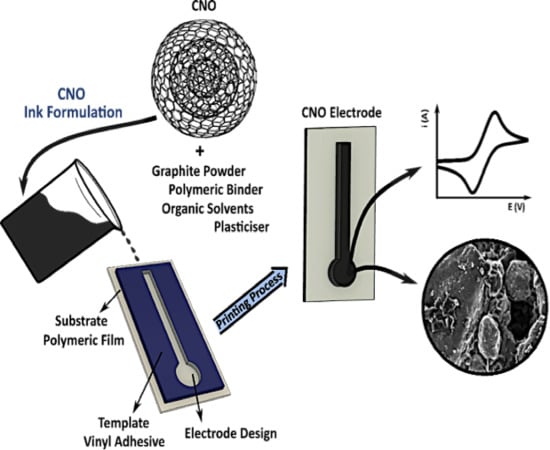
Electrochemical Properties of Screen-Printed Carbon Nano-Onion Electrodes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural and Chemical Properties
2.2. Elemental Composition
2.3. Raman Spectroscopy
2.4. Electrochemical Properties
2.5. Biomolecule Detection
3. Materials and Methods
3.1. Materials
3.2. Instrumentation
3.3. CNO/GRT Optimised Ink Formulation
3.4. Substrate Surface Pretreatment
3.5. CNO/GRT SPE Printing Process
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, J.; Tian, B.; Nascimento, V.B.; Angnes, L. Performance of screen-printed carbon electrodes fabricated from different carbon inks. Electrochim. Acta 1998, 43, 3459–3465. [Google Scholar] [CrossRef]
- Wang, J.; Musameh, M. Carbon nanotube screen-printed electrochemical sensors. Analyst 2004, 129, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, N.; Tiwari, I. Recent build outs in electroanalytical biosensors based on carbon-nanomaterial modified screen printed electrode platforms. Anal. Methods 2017, 9, 3895–3907. [Google Scholar] [CrossRef]
- Li, M.; Li, Y.-T.; Li, D.-W.; Long, Y.-T. Recent developments and applications of screen-printed electrodes in environmental assays—A review. Anal. Chim. Acta 2012, 734, 31–44. [Google Scholar] [CrossRef]
- Metters, J.P.; Kadara, R.O.; Banks, C.E. New directions in screen printed electroanalytical sensors: An overview of recent developments. Analyst 2011, 136, 1067–1076. [Google Scholar] [CrossRef]
- Arduini, F.; Micheli, L.; Moscone, D.; Palleschi, G.; Piermarini, S.; Ricci, F.; Volpe, G. Electrochemical biosensors based on nanomodified screen-printed electrodes: Recent applications in clinical analysis. TrAC Trends Anal. Chem. 2016, 79, 114–126. [Google Scholar] [CrossRef] [Green Version]
- Taleat, Z.; Khoshroo, A.; Mazloum-Ardakani, M. Screen-printed electrodes for biosensing: A review (2008–2013). Microchim. Acta 2014, 181, 865–891. [Google Scholar] [CrossRef]
- de Eguilaz, M.R.; Cumba, L.R.; Forster, R.J. Electrochemical detection of viruses and antibodies: A mini review. Electrochem. Commun. 2020, 116, 106762. [Google Scholar] [CrossRef]
- Forster, R.J.; Cadogan, A.; Telting Diaz, M.; Diamond, D.; Harris, S.J.; McKervey, M.A. Calixarenes as active agents for chemical sensors. Sens. Actuators B Chem. 1991, 4, 325–331. [Google Scholar] [CrossRef]
- Metters, J.P.; Gomez-Mingot, M.; Iniesta, J.; Kadara, R.O.; Banks, C.E. The fabrication of novel screen printed single-walled carbon nanotube electrodes: Electroanalytical applications. Sens. Actuators B Chem. 2013, 177, 1043–1052. [Google Scholar] [CrossRef]
- Moreno, M.; Arribas, A.S.; Bermejo, E.; Chicharro, M.; Zapardiel, A.; Rodríguez, M.C.; Jalit, Y.; Rivas, G.A. Selective detection of dopamine in the presence of ascorbic acid using carbon nanotube modified screen-printed electrodes. Talanta 2010, 80, 2149–2156. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, D.; Huang, J.; You, T. Simultaneous determination of dopamine, ascorbic acid and uric acid at electrochemically reduced graphene oxide modified electrode. Sens. Actuators B Chem. 2014, 193, 166–172. [Google Scholar] [CrossRef]
- Silva, B.V.M.; Cavalcanti, I.T.; Silva, M.M.S.; Dutra, R.F. A carbon nanotube screen-printed electrode for label-free detection of the human cardiac troponin T. Talanta 2013, 117, 431–437. [Google Scholar] [CrossRef]
- Bonanni, A.; Esplandiu, M.J.; del Valle, M. Impedimetric genosensors employing COOH-modified carbon nanotube screen-printed electrodes. Biosens. Bioelectron. 2009, 24, 2885–2891. [Google Scholar] [CrossRef] [PubMed]
- Cinti, S.; Arduini, F. Graphene-based screen-printed electrochemical (bio)sensors and their applications: Efforts and criticisms. Biosens. Bioelectron. 2017, 89, 107–122. [Google Scholar] [CrossRef] [PubMed]
- Ugarte, D. Curling and closure of graphitic networks under electron-beam irradiation. Nature 1992, 359, 707–709. [Google Scholar] [CrossRef]
- Lin, L.; Peng, H.; Liu, Z. Synthesis challenges for graphene industry. Nat. Mater. 2019, 18, 520–524. [Google Scholar] [CrossRef]
- Bhuyan, M.S.A.; Uddin, M.N.; Islam, M.M.; Bipasha, F.A.; Hossain, S.S. Synthesis of graphene. Int. Nano Lett. 2016, 6, 65–83. [Google Scholar] [CrossRef] [Green Version]
- Camisasca, A.; Giordani, S. Carbon nano-onions in biomedical applications: Promising theranostic agents. Inorg. Chim. Acta 2017, 468, 67–76. [Google Scholar] [CrossRef]
- Giordani, S.; Maffeis, V.; Camisasca, A. Carbon Nano-onions: A Valuable Class of Carbon Nanomaterials in Biomedicine. Curr. Med. Chem. 2019, 26, 6915–6929. [Google Scholar] [CrossRef]
- Lettieri, S.; Camisasca, A.; d’Amora, M.; Diaspro, A.; Uchida, T.; Nakajima, Y.; Yanagisawa, K.; Maekawa, T.; Giordani, S. Far-red fluorescent carbon nano-onions as a biocompatible platform for cellular imaging. RSC Adv. 2017, 7, 45676–45681. [Google Scholar] [CrossRef] [Green Version]
- Zeiger, M.; Jäckel, N.; Mochalin, V.N.; Presser, V. Review: Carbon onions for electrochemical energy storage. J. Mater. Chem. A 2016, 4, 3172–3196. [Google Scholar] [CrossRef] [Green Version]
- Camisasca, A.; Sacco, A.; Brescia, R.; Giordani, S. Boron/Nitrogen-Codoped Carbon Nano-Onion Electrocatalysts for the Oxygen Reduction Reaction. ACS Appl. Nano Mater. 2018, 1, 5763–5773. [Google Scholar] [CrossRef]
- Bartelmess, J.; Giordani, S. Carbon nano-onions (multi-layer fullerenes): Chemistry and applications. Beilstein J. Nanotechnol. 2014, 5, 1980–1998. [Google Scholar] [CrossRef] [PubMed]
- Bartolome, J.P.; Echegoyen, L.; Fragoso, A. Reactive Carbon Nano-Onion Modified Glassy Carbon Surfaces as DNA Sensors for Human Papillomavirus Oncogene Detection with Enhanced Sensitivity. Anal. Chem. 2015, 87, 6744–6751. [Google Scholar] [CrossRef]
- Mohapatra, J.; Ananthoju, B.; Nair, V.; Mitra, A.; Bahadur, D.; Medhekar, N.V.; Aslam, M. Enzymatic and non-enzymatic electrochemical glucose sensor based on carbon nano-onions. Appl. Surf. Sci. 2018, 442, 332–341. [Google Scholar] [CrossRef]
- Sok, V.; Fragoso, A. Preparation and characterization of alkaline phosphatase, horseradish peroxidase, and glucose oxidase conjugates with carboxylated carbon nano-onions. Prep. Biochem. Biotechnol. 2018, 48, 136–143. [Google Scholar] [CrossRef]
- Zuaznabar-Gardona, J.C.; Fragoso, A. A wide-range solid state potentiometric pH sensor based on poly-dopamine coated carbon nano-onion electrodes. Sens. Actuators B Chem. 2018, 273, 664–671. [Google Scholar] [CrossRef]
- Singh, V. Natural source derived carbon nano-onions as electrode material for sensing applications. Diam. Relat. Mater. 2018, 87, 202–207. [Google Scholar] [CrossRef]
- Ibáñez-Redín, G.; Furuta, R.H.M.; Wilson, D.; Shimizu, F.M.; Materon, E.M.; Arantes, L.M.R.B.; Melendez, M.E.; Carvalho, A.L.; Reis, R.M.; Chaur, M.N.; et al. Screen-printed interdigitated electrodes modified with nanostructured carbon nano-onion films for detecting the cancer biomarker CA19-9. Mater. Sci. Eng. C 2019, 99, 1502–1508. [Google Scholar] [CrossRef]
- Camisasca, A.; Giordani, S. Surfactant-mediated dispersions of carbon nano-onions in aqueous solution. Nano Express 2020, 1, 10018. [Google Scholar] [CrossRef]
- Ren, D.; Zheng, S.; Wu, F.; Yang, W.; Liu, Z.; Yang, M. Formation and evolution of the carbon black network in polyethylene/carbon black composites: Rheology and conductivity properties. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Sandler, J.K.W.; Kirk, J.E.; Kinloch, I.A.; Shaffer, M.S.P.; Windle, A.H. Ultra-low electrical percolation threshold in carbon-nanotube-epoxy composites. Polymer (Guildf) 2003, 44, 5893–5899. [Google Scholar] [CrossRef]
- Choi, H.-J.; Kim, M.S.; Ahn, D.; Yeo, S.Y.; Lee, S. Electrical percolation threshold of carbon black in a polymer matrix and its application to antistatic fibre. Sci. Rep. 2019, 9, 6338. [Google Scholar] [CrossRef] [Green Version]
- Park, S.-H.; Hwang, J.; Park, G.-S.; Ha, J.-H.; Zhang, M.; Kim, D.; Yun, D.-J.; Lee, S.; Lee, S.H. Modeling the electrical resistivity of polymer composites with segregated structures. Nat. Commun. 2019, 10, 2537. [Google Scholar] [CrossRef] [Green Version]
- Hu, N.; Karube, Y.; Yan, C.; Masuda, Z.; Fukunaga, H. Tunneling effect in a polymer/carbon nanotube nanocomposite strain sensor. Acta Mater. 2008, 56, 2929–2936. [Google Scholar] [CrossRef] [Green Version]
- Akhavan, O. The effect of heat treatment on formation of graphene thin films from graphene oxide nanosheets. Carbon N. Y. 2010, 48, 509–519. [Google Scholar] [CrossRef]
- Kundu, S.; Wang, Y.; Xia, W.; Muhler, M. Thermal Stability and Reducibility of Oxygen-Containing Functional Groups on Multiwalled Carbon Nanotube Surfaces: A Quantitative High-Resolution XPS and TPD/TPR Study. J. Phys. Chem. C 2008, 112, 16869–16878. [Google Scholar] [CrossRef]
- Haubner, K.; Murawski, J.; Olk, P.; Eng, L.M.; Ziegler, C.; Adolphi, B.; Jaehne, E. The Route to Functional Graphene Oxide. ChemPhysChem 2010, 11, 2131–2139. [Google Scholar] [CrossRef] [PubMed]
- Çopuroğlu, M.; Sezen, H.; Opila, R.L.; Suzer, S. Band-Bending at Buried SiO2/Si Interface as Probed by XPS. ACS Appl. Mater. Interfaces 2013, 5, 5875–5881. [Google Scholar] [CrossRef]
- Döscher, H.; Brückner, S.; Dobrich, A.; Höhn, C.; Kleinschmidt, P.; Hannappel, T. Surface preparation of Si(100) by thermal oxide removal in a chemical vapor environment. J. Cryst. Growth 2011, 315, 10–15. [Google Scholar] [CrossRef]
- Wu, G.; Yu, Y.; Cheng, X.; Zhang, Y. Preparation and surface modification mechanism of silica aerogels via ambient pressure drying. Mater. Chem. Phys. 2011, 129, 308–314. [Google Scholar] [CrossRef]
- Tan, D.; Ma, Z.; Xu, B.; Dai, Y.; Ma, G.; He, M.; Jin, Z.; Qiu, J. Surface passivated silicon nanocrystals with stable luminescence synthesized by femtosecond laser ablation in solution. Phys. Chem. Chem. Phys. 2011, 13, 20255–20261. [Google Scholar] [CrossRef] [PubMed]
- Llansola Portolés, M.J.; Rodriguez Nieto, F.; Soria, D.B.; Amalvy, J.I.; Peruzzo, P.J.; Mártire, D.O.; Kotler, M.; Holub, O.; Gonzalez, M.C. Photophysical Properties of Blue-Emitting Silicon Nanoparticles. J. Phys. Chem. C 2009, 113, 13694–13702. [Google Scholar] [CrossRef] [Green Version]
- Barr, T.L. An XPS study of Si as it occurs in adsorbents, catalysts, and thin films. Appl. Surf. Sci. 1983, 15, 1–35. [Google Scholar] [CrossRef]
- Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S.; Cançado, L.G.; Jorio, A.; Saito, R. Studying disorder in graphite-based systems by Raman spectroscopy. Phys. Chem. Chem. Phys. 2007, 9, 1276–1290. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman Spectrum of Graphene and Graphene Layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, A.; Robertson, J.; Reich, S.; Thomsen, C. Raman spectroscopy of graphite. Philos. Trans. R. Soc. London. Ser. A Math. Phys. Eng. Sci. 2004, 362, 2271–2288. [Google Scholar] [CrossRef]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron–phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Obraztsova, E.D.; Fujii, M.; Hayashi, S.; Kuznetsov, V.L.; Butenko, Y.V.; Chuvilin, A.L. Raman identification of onion-like carbon. Carbon N. Y. 1998, 36, 821–826. [Google Scholar] [CrossRef]
- Roy, D.; Chhowalla, M.; Wang, H.; Sano, N.; Alexandrou, I.; Clyne, T.W.; Amaratunga, G.A.J. Characterisation of carbon nano-onions using Raman spectroscopy. Chem. Phys. Lett. 2003, 373, 52–56. [Google Scholar] [CrossRef]
- Forster, R.J.; Cumba, L.R. 29-Optimizing glucose sensing for diabetes monitoring. In Woodhead Publishing Series in Electronic and Optical Materials; Pal, K., Kraatz, H.-B., Khasnobish, A., Bag, S., Banerjee, I., Kuruganti, U.B.T.-B., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 765–778. ISBN 978-0-08-102420-1. [Google Scholar]
- Nicholson, R.S.; Shain, I. Theory of Stationary Electrode Polarography. Single Scan and Cyclic Methods Applied to Reversible, Irreversible, and Kinetic Systems. Anal. Chem. 1964, 36, 706–723. [Google Scholar] [CrossRef]
- Choudry, N.A.; Kampouris, D.K.; Kadara, R.O.; Banks, C.E. Disposable highly ordered pyrolytic graphite-like electrodes: Tailoring the electrochemical reactivity of screen printed electrodes. Electrochem. Commun. 2010, 12, 6–9. [Google Scholar] [CrossRef]
- Forster, R.J.; Keyes, T.E. 6-Ultramicroelectrodes. In Handbook of Electrochemistry; Zoski, C., Ed.; Elsevier: Amsterdam, The Netherlands, 2007; ISBN 978-0-444-51958-0. [Google Scholar]
- Nichkova, M.; Wynveen, P.M.; Marc, D.T.; Huisman, H.; Kellermann, G.H. Validation of an ELISA for urinary dopamine: Applications in monitoring treatment of dopamine-related disorders. J. Neurochem. 2013, 125, 724–735. [Google Scholar] [CrossRef]
- Klein, M.O.; Battagello, D.S.; Cardoso, A.R.; Hauser, D.N.; Bittencourt, J.C.; Correa, R.G. Dopamine: Functions, Signaling, and Association with Neurological Diseases. Cell. Mol. Neurobiol. 2019, 39, 31–59. [Google Scholar] [CrossRef]
- Rowley-Neale, S.J.; Brownson, D.A.C.; Smith, G.; Banks, C.E. Graphene Oxide Bulk-Modified Screen-Printed Electrodes Provide Beneficial Electroanalytical Sensing Capabilities. Biosensors 2020, 10, 27. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, S.; Taher, M.A.; Beitollahi, H. Treated Screen Printed Electrodes Based on Electrochemically Reduced Graphene Nanoribbons for the Sensitive Voltammetric Determination of Dopamine in the Presence of Uric Acid. Electroanalysis 2020. [Google Scholar] [CrossRef]
- How, G.T.S.; Pandikumar, A.; Ming, H.N.; Ngee, L.H. Highly exposed {001} facets of titanium dioxide modified with reduced graphene oxide for dopamine sensing. Sci. Rep. 2014, 4, 5044. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.-M.; Xu, P.-L.; Zeng, Q.; Liu, Y.-M.; Liao, X.; Hou, M.-F. Magnetism-assisted modification of screen printed electrode with magnetic multi-walled carbon nanotubes for electrochemical determination of dopamine. Mater. Sci. Eng. C 2017, 74, 62–69. [Google Scholar] [CrossRef] [Green Version]
- Baccarin, M.; Rowley-Neale, S.J.; Cavalheiro, É.T.G.; Smith, G.C.; Banks, C.E. Nanodiamond based surface modified screen-printed electrodes for the simultaneous voltammetric determination of dopamine and uric acid. Microchim. Acta 2019, 186, 200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choo, S.-S.; Kang, E.-S.; Song, I.; Lee, D.; Choi, J.-W.; Kim, T.-H. Electrochemical Detection of Dopamine Using 3D Porous Graphene Oxide/Gold Nanoparticle Composites. Sensors (Basel) 2017, 17, 861. [Google Scholar] [CrossRef] [PubMed]
- Wiench, P.; González, Z.; Menéndez, R.; Grzyb, B.; Gryglewicz, G. Beneficial impact of oxygen on the electrochemical performance of dopamine sensors based on N-doped reduced graphene oxides. Sens. Actuators B Chem. 2018, 257, 143–153. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |







| Material | Wt./Wt.% |
|---|---|
| Polyhydroxyethers | 10 |
| Di(propylene glycol) methyl ether | 55 |
| Poly(dimethylsiloxane-co-methylphenylsiloxane) | 4 |
| Carbon nano-onions | 24 |
| Graphite | 7 |
| Element | Estimated Weight% | EDS Weight% |
|---|---|---|
| C | 93.4 | 80.6 |
| O | 5.2 | 18.1 |
| Si | 1.4 | 1.3 |
| Sample | C (at. %) | O (at. %) | Si (at. %) |
|---|---|---|---|
| p-CNOs | 93.7 | 5.6 | 0.8 |
| Graphite | 96.8 | 2.9 | 0.3 |
| CNO/GRT SPEs | 79.5 | 17.8 | 2.7 |
| Electrode | Linear Working Range (μM) | Dopamine (DA) LOD (µM) | Electrochemical Method | Reference |
|---|---|---|---|---|
| Graphene oxide nanoribbons/SPE | 0.5–300.0 | 0.15 | Differential pulse voltammetry | [60] |
| Reduced graphene oxide/TiO2 {001}/GCE | 2.0–60.0 | 6.00 | Differential pulse voltammetry | [61] |
| Magnetic multi-walled carbon nanotubes/SPE | 5.0–180.0 | 0.43 | Square wave voltammetry | [62] |
| Nanodiamonds/SPE | 2.0–100.0 | 0.57 | Differential pulse voltammetry | [63] |
| 3D porous graphene oxide-gold nanoparticle/ITO | 0.1–30.0 | 1.28 | Cyclic Voltammetry | [64] |
| Nitrogen-doped reduced graphene oxides (150-2)/GCE | 3.0–70.0 | 1.50 | Differential pulse voltammetry | [65] |
| CNO/GRT SPE | 10.0–99.9 | 0.92 | Cyclic Voltammetry | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cumba, L.R.; Camisasca, A.; Giordani, S.; Forster, R.J. Electrochemical Properties of Screen-Printed Carbon Nano-Onion Electrodes. Molecules 2020, 25, 3884. https://doi.org/10.3390/molecules25173884
Cumba LR, Camisasca A, Giordani S, Forster RJ. Electrochemical Properties of Screen-Printed Carbon Nano-Onion Electrodes. Molecules. 2020; 25(17):3884. https://doi.org/10.3390/molecules25173884
Chicago/Turabian StyleCumba, Loanda R., Adalberto Camisasca, Silvia Giordani, and Robert J. Forster. 2020. "Electrochemical Properties of Screen-Printed Carbon Nano-Onion Electrodes" Molecules 25, no. 17: 3884. https://doi.org/10.3390/molecules25173884
APA StyleCumba, L. R., Camisasca, A., Giordani, S., & Forster, R. J. (2020). Electrochemical Properties of Screen-Printed Carbon Nano-Onion Electrodes. Molecules, 25(17), 3884. https://doi.org/10.3390/molecules25173884