Applications and Limitations of Dendrimers in Biomedicine
Abstract
:1. Introduction
2. Biomedical Dendrimer Profile-Cytotoxicity
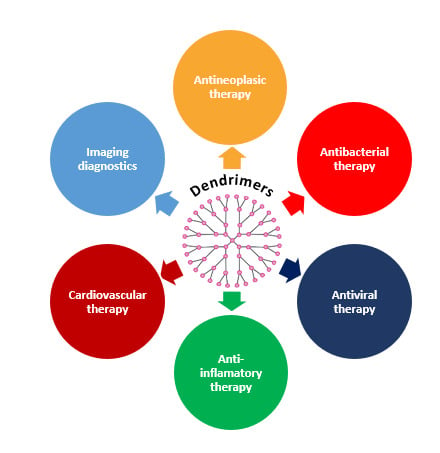
3. Biomedical Applications of Dendrimers
3.1. Dendrimers in Drug Therapy
3.1.1. Dendrimers in Antineoplastic Therapy
Poly(amidoamine) Dendrimers (PAMAM)
Poly(propylene imine) Dendrimers (PPI)
Poly-L-lysine dendrimers (PLL)
Tryptophan-rich Peptide Dendrimers (TRPD)
Phosphorus Dendrimers
3.1.2. Dendrimers in Anti-Inflammatory Therapy
3.1.3. Dendrimers in Antibacterial Therapy
3.1.4. Dendrimers in Antiviral Therapy
3.1.5. Dendrimers in Cardiovascular Therapy
3.2. Dendrimers in Imaging Diagnostics
4. Toxicity Reports Regarding Dendrimers
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Carmo, D.R.; Silveira, S.F.T.; Laurentiz, S.R.; Bicalho, M.L.; Filho, D.L.; Paim, L.L. Synthesis and a preliminary characterization of poly(propylene)imine hexadecylamine dendrimer (DAB-Am-16) modified with methyl acrylate. Am. Chem. Sci. J. 2013, 3, 314–324. [Google Scholar] [CrossRef]
- Augustus, E.N.; Allen, E.T.; Nimibofa, A.; Donbebe, W. A review of synthesis, characterization and applications of functionalized dendrimers. Am. J. Polym. Sci. 2017, 7, 8–14. [Google Scholar] [CrossRef]
- Lyu, Z.; Ding, L.; Huang, A.Y.T.; Kao, C.L.; Peng, L. Poly(amidoamine)dendrimers: Covalent and supramolecular synthesis. Mater. Today Chem. 2019, 13, 34–48. [Google Scholar] [CrossRef]
- Sohail, I.; Bhatti, I.A.; Ashar, A.; Sarim, F.M.; Mohsin, M.; Naveed, R.; Yasir, M.; Iqbal, M.; Nazir, A. Polyamidoamine (PAMAM) dendrimers synthesis, characterization and adsorptive removal of nickel ions from aqueous solution. J. Mater. Res. Technol. 2020, 9, 498–506. [Google Scholar] [CrossRef]
- Pandita, D.; Poonia, N.; Kumar, S.; Lather, V.; Madaan, K. Dendrimers in drug delivery and targeting: Drug-dendrimer interactions and toxicity issues. J. Pharm. Bioallied Sci. 2014, 6, 139–150. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhao, L.; Li, Y.; Xu, T. Design of biocompatible dendrimers for cancer diagnosis and therapy: Current status and future perspectives. Chem. Soc. Rev. 2011, 40, 2673–2703. [Google Scholar] [CrossRef] [PubMed]
- Sherje, A.P.; Jadhav, M.; Dravyakar, B.R.; Kadam, D. Dendrimers: A versatile nanocarrier for drug delivery and targeting. Int. J. Pharm. 2018, 548, 707–720. [Google Scholar] [CrossRef] [PubMed]
- Cojocaru, F.D.; Botezat, D.; Gardikiotis, I.; Uritu, C.M.; Dodi, G.; Trandafir, L.; Rezus, C.; Rezus, E.; Tamba, B.I.; Mihai, C.T. Nanomaterials designed for antiviral drug delivery transport across biological barriers. Pharmaceutics 2020, 12, 171. [Google Scholar] [CrossRef] [Green Version]
- Patel, V.; Rajani, C.; Paul, D.; Borisa, P.; Rajpoot, K.; Youngren-Ortiz, S.R.; Tekade, R.K. Dendrimers as novel drug-delivery system and its applications. In Drug Delivery Systems; Elsevier: Cambridge, MA, USA, 2020; pp. 333–392. ISBN 9780128145081. [Google Scholar]
- Shadrack, D.; Swai, H.; Munissi, J.; Mubofu, E.; Nyandoro, S. Polyamidoamine dendrimers for enhanced solubility of small molecules and other desirable properties for site specific delivery: Insights from experimental and computational studies. Molecules 2018, 23, 1419. [Google Scholar] [CrossRef] [Green Version]
- Shadrack, D.; Mubofu, E.; Nyandoro, S. Synthesis of polyamidoamine dendrimer for encapsulating tetramethylscutellarein for potential bioactivity enhancement. Int. J. Mol. Sci. 2015, 16, 26363–26377. [Google Scholar] [CrossRef]
- Prajapati, R.N.; Tekade, R.K.; Gupta, U.; Gajbhiye, V.; Jain, N.K. Dendimer-mediated solubilization, formulation development and in vitro-in vivo assessment of piroxicam. Mol. Pharm. 2009, 6, 940–950. [Google Scholar] [CrossRef]
- Otto, D.P.; de Villiers, M.M. Poly(amidoamine) dendrimers as a pharmaceutical excipient. Are We There yet? J. Pharm. Sci. 2018, 107, 75–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, H.J.; Bugno, J.; Lee, S.R.; Hong, S. Dendrimer-based nanocarriers: A versatile platform for drug delivery. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1409. [Google Scholar] [CrossRef] [PubMed]
- Bello, M.; Fragoso-Vazquez, J.; Correa-Basurto, J. Theoretical studies for dendrimer-based drug delivery. Curr. Pharm. Des. 2017, 23. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.S. Dendrimers for drug delivery. Molecules 2018, 23, 938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, J.; Hu, K.; Cheng, Y. Tailoring the dendrimer core for efficient gene delivery. Acta Biomater. 2016, 35, 1–11. [Google Scholar] [CrossRef]
- Kowalski, P.S.; Rudra, A.; Miao, L.; Anderson, D.G. Delivering the messenger: Advances in technologies for therapeutic mRNA delivery. Mol. Ther. 2019, 27, 710–728. [Google Scholar] [CrossRef] [Green Version]
- Marquez-Miranda, V.; Araya-Duran, I.; Gonzalez-Nilo, F.D. Multiscale molecular simulations applied to nucleic acid-dendrimer interactions studies. Curr. Pharm. Des. 2017, 23. [Google Scholar] [CrossRef]
- Li, D.; Wen, S.; Shi, X. Dendrimer-entrapped metal colloids as imaging agents. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 678–690. [Google Scholar] [CrossRef]
- Xiao, T.; Li, D.; Shi, X.; Shen, M. PAMAM Dendrimer-based nanodevices for nuclear medicine applications. Macromol. Biosci. 2020, 20. [Google Scholar] [CrossRef]
- Anwaier, G.; Chen, C.; Cao, Y.; Qi, R. A review of molecular imaging of atherosclerosis and the potential application of dendrimer in imaging of plaque. Int. J. Nanomed. 2017, 12, 7681–7693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumari, P.; Ghosh, B.; Biswas, S. Nanocarriers for cancer-targeted drug delivery. J. Drug Target. 2016, 24, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K.; Gothwal, A.; Kesharwani, P.; Alsaab, H.; Iyer, A.K.; Gupta, U. Dendrimer nanoarchitectures for cancer diagnosis and anticancer drug delivery. Drug Discov. Today 2017, 22, 314–326. [Google Scholar] [CrossRef] [PubMed]
- Soni, N.; Tekade, M.; Kesharwani, P.; Bhattacharya, P.; Maheshwari, R.; Dua, K.; Hansbro, P.M.; Tekade, R.K. Recent advances in oncological submissions of dendrimer. Curr. Pharm. Des. 2017, 23. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Park, E.J.; Na, D.H. Recent progress in dendrimer-based nanomedicine development. Arch. Pharm. Res. 2018, 41, 571–582. [Google Scholar] [CrossRef]
- Yu, M.; Jie, X.; Xu, L.; Chen, C.; Shen, W.; Cao, Y.; Lian, G.; Qi, R. Recent advances in dendrimer research for cardiovascular diseases. Biomacromolecules 2015, 16, 2588–2598. [Google Scholar] [CrossRef]
- Fruchon, S.; Poupot, R. The ABP dendrimer, a drug-candidate against inflammatory diseases that triggers the activation of interleukin-10 producing immune cells. Molecules 2018, 23, 1272. [Google Scholar] [CrossRef] [Green Version]
- Shaunak, S. Perspective: Dendrimer drugs for infection and inflammation. Biochem. Biophys. Res. Commun. 2015, 468, 435–441. [Google Scholar] [CrossRef]
- Hossen, S.; Hossain, M.K.; Basher, M.K.; Mia, M.N.H.; Rahman, M.T.; Uddin, M.J. Smart nanocarrier-based drug delivery systems for cancer therapy and toxicity studies: A review. J. Adv. Res. 2019, 15, 1–18. [Google Scholar] [CrossRef]
- Santos, A.; Veiga, F.; Figueiras, A. Dendrimers as pharmaceutical excipients: Synthesis, properties, toxicity and biomedical applications. Materials 2019, 13, 65. [Google Scholar] [CrossRef] [Green Version]
- Sowinska, M.; Urbanczyk-Lipkowska, Z. Advances in the chemistry of dendrimers. New J. Chem. 2014, 38, 2168–2203. [Google Scholar] [CrossRef]
- Caminade, A.-M.; Majoral, J.-P. Which dendrimer to attain the desired properties? Focus on Phosphorhydrazone Dendrimers. Molecules 2018, 23, 622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbasi, E.; Aval, S.F.; Akbarzadeh, A.; Milani, M.; Nasrabadi, H.T.; Joo, S.W.; Hanifehpour, Y.; Nejati-Koshki, K.; Pashaei-Asl, R. Dendrimers: Synthesis, applications, and properties. Nanoscale Res. Lett. 2014, 9, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulthe, S.S.; Choudhari, Y.M.; Inamdar, N.N.; Mourya, V. Polymeric micelles: Authoritative aspects for drug delivery. Des. Monomers Polym. 2012, 15, 465–521. [Google Scholar] [CrossRef]
- Elsabahy, M.; Wooley, K.L. Design of polymeric nanoparticles for biomedical delivery applications. Chem. Soc. Rev. 2012, 41, 2545–2561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fox, L.J.; Richardson, R.M.; Briscoe, W.H. PAMAM dendrimer-cell membrane interactions. Adv. Colloid Interface Sci. 2018, 257, 1–18. [Google Scholar] [CrossRef]
- Bharali, D.J.; Khalil, M.; Gurbuz, M.; Simone, T.M.; Mousa, S.A. Nanoparticles and cancer therapy: A concise review with emphasis on dendrimers. Int. J. Nanomed. 2009, 4, 1–7. [Google Scholar]
- Abd-El-Aziz, A.S.; Agatemor, C. Emerging opportunities in the biomedical applications of dendrimers. J. Inorg. Organomet. Polym. Mater. 2018, 28, 369–382. [Google Scholar] [CrossRef]
- Janaszewska, A.; Lazniewska, J.; Trzepiński, P.; Marcinkowska, M.; Klajnert-Maculewicz, B. Cytotoxicity of dendrimers. Biomolecules 2019, 9, 330. [Google Scholar] [CrossRef] [Green Version]
- Tomalia, D.A.; Huang, B.; Swanson, D.R.; Brothers, H.M.; Klimash, J.W. Structure control within poly(amidoamine) dendrimers: Size, shape and regio-chemical mimicry of globular proteins. Tetrahedron 2003, 59, 3799–3813. [Google Scholar] [CrossRef]
- Jevprasesphant, R.; Penny, J.; Jalal, R.; Attwood, D.; McKeown, N.B.; D’Emanuele, A. The influence of surface modification on the cytotoxicity of PAMAM dendrimers. Int. J. Pharm. 2003, 252, 263–266. [Google Scholar] [CrossRef]
- Malik, N.; Wiwattanapatapee, R.; Klopsch, R.; Lorenz, K.; Frey, H.; Weener, J.W.; Meijer, E.W.; Paulus, W.; Duncan, R. Dendrimers: Relationship between structure and biocompatibility in vitro, and preliminary studies on the biodistribution of 125I-labelled polyamidoamine dendrimers in vivo. J. Control. Release 2000, 65, 133–148. [Google Scholar] [CrossRef]
- Ciolkowski, M.; Petersen, J.F.; Ficker, M.; Janaszewska, A.; Christensen, J.B.; Klajnert, B.; Bryszewska, M. Surface modification of PAMAM dendrimer improves its biocompatibility. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 815–817. [Google Scholar] [CrossRef] [PubMed]
- Beddoes, C.M.; Case, C.P.; Briscoe, W.H. Understanding nanoparticle cellular entry: A physicochemical perspective. Adv. Colloid Interface Sci. 2015, 218, 48–68. [Google Scholar] [CrossRef]
- Pandurangan, A.K.; Kanagesan, S.; Narayanaswamy, R.; Esa, N.M.; Parasuraman, P. Nanobiomaterial-based delivery of drugs in various cancer therapies: Classifying the mechanisms of action (using biochemical and molecular biomarkers). In Nanobiomaterials in Cancer Therapy: Applications of Nanobiomaterials; Elsevier Inc.: Norwich, NY, USA, 2016; pp. 331–365. ISBN 9780323428866. [Google Scholar]
- Farooq, M.A.; Aquib, M.; Farooq, A.; Khan, D.H.; Maviah, M.B.J.; Filli, M.S.; Kesse, S.; Boakye-Yiadom, K.O.; Mavlyanova, R.; Parveen, A.; et al. Recent progress in nanotechnology-based novel drug delivery systems in designing of cisplatin for cancer therapy: An overview. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1674–1692. [Google Scholar] [CrossRef] [Green Version]
- Wrobel, D.; Müllerová, M.; Strašák, T.; Růžička, K.; Fulem, M.; Kubíková, R.; Bryszewska, M.; Klajnert-Maculewicz, B.; Malý, J. Glucose-modified carbosilane dendrimers: Interaction with model membranes and human serum albumin. Int. J. Pharm. 2020, 579, 119138. [Google Scholar] [CrossRef]
- Boas, U.; Christensen, J.B.; HeegaardJ, P.M.H. Dendrimers in medicine and biotechnology: New molecular tools. RCS Publ. 2006, 2, 28–61. [Google Scholar]
- Yang, W.; Cheng, Y.; Xu, T.; Wang, X.; Wen, L.P. Targeting cancer cells with biotin-dendrimer conjugates. Eur. J. Med. Chem. 2009, 44, 862–868. [Google Scholar] [CrossRef]
- Shao, N.; Su, Y.; Hu, J.; Zhang, J.; Zhang, H.; Cheng, Y. Comparison of generation 3 polyamidoamine dendrimer and generation 4 polypropylenimine dendrimer on drug loading, complex structure, release behavior, and cytotoxicity. Int. J. Nanomed. 2011, 6, 3361–3372. [Google Scholar]
- Pourianazar, N.T.; Mutlu, P.; Gunduz, U. Bioapplications of poly(amidoamine) (PAMAM) dendrimers in nanomedicine. J. Nanopart. Res. 2014, 16, 1–38. [Google Scholar] [CrossRef]
- Gardikis, K.; Micha-Screttas, M.; Demetzos, C.; Steele, B.R. Dendrimers and the development of new complex nanomaterials for biomedical applications. Curr. Med. Chem. Curr. Med. Chem. 2012, 19, 4913–4928. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.S.; Bae, Y.M.; Choi, H.; Kong, B.; Choi, I.S.; Choi, J.S. Synthesis of PAMAM dendrimer derivatives with enhanced buffering capacity and remarkable gene transfection efficiency. Bioconjug. Chem. 2011, 22, 1046–1055. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.P.; Davoren, M.; Byrne, H.J. In vitro mammalian cytotoxicological study of PAMAM dendrimers—Towards quantitative structure activity relationships. Toxicol. Vitr. 2010, 24, 169–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukherjee, S.P.; Lyng, F.M.; Garcia, A.; Davoren, M.; Byrne, H.J. Mechanistic studies of in vitro cytotoxicity of poly(amidoamine) dendrimers in mammalian cells. Toxicol. Appl. Pharmacol. 2010, 248, 259–268. [Google Scholar] [CrossRef] [Green Version]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Zha, J.; Wang, Y.; Liu, W.; Yang, X.; Yu, P. Tissue damage-associated “danger signals” influence T-cell responses that promote the progression of preneoplasia to cancer. Cancer Res. 2013, 73, 629–639. [Google Scholar] [CrossRef] [Green Version]
- Zitvogel, L.; Galluzzi, L.; Smyth, M.J.; Kroemer, G. Mechanism of action of conventional and targeted anticancer therapies: Reinstating immunosurveillance. Immunity 2013, 39, 74–88. [Google Scholar] [CrossRef] [Green Version]
- Gerber, D.E. Targeted therapies: A new generation of cancer treatments. Am. Fam. Physician 2008, 77, 311–319. [Google Scholar]
- Cleeland, C.S.; Allen, J.D.; Roberts, S.A.; Brell, J.M.; Giralt, S.A.; Khakoo, A.Y.; Kirch, R.A.; Kwitkowski, V.E.; Liao, Z.; Skillings, J. Reducing the toxicity of cancer therapy: Recognizing needs, taking action. Nat. Rev. Clin. Oncol. 2012, 9, 471–478. [Google Scholar] [CrossRef]
- Morikawa, A. Comparison of properties among dendritic and hyperbranched poly(ether ether ketone)s and linear poly(ether ketone)s. Molecules 2016, 21, 219. [Google Scholar] [CrossRef] [Green Version]
- Castro, R.I.; Forero-Doria, O.; Guzmán, L. Perspectives of dendrimer-based nanoparticles in cancer therapy. An. Acad. Bras. Cienc. 2018, 90, 2331–2346. [Google Scholar] [CrossRef] [Green Version]
- Medina, S.H.; El-Sayed, M.E.H. Dendrimers as carriers for delivery of chemotherapeutic agents. Chem. Rev. 2009, 109, 3141–3157. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, M.; Rajabi, M.; Mousa, S.A. Multifunctional nanomaterials and their applications in drug delivery and cancer therapy. Nanomaterials 2015, 5, 1690–1703. [Google Scholar] [CrossRef] [PubMed]
- Noriega-Luna, B.; Godínez, L.A.; Rodríuez, F.J.; Rodríguez, A.; Zaldívar-Lelo de Larrea, G.; Sosa-Ferreyra, C.F.; Mercado-Curiel, R.F.; Manríquez, J.; Bustos, E. Applications of dendrimers in drug delivery agents, diagnosis, therapy, and detection. J. Nanomater. 2014, 2014, 19. [Google Scholar] [CrossRef] [Green Version]
- Choudhary, S.; Gupta, L.; Rani, S.; Dave, K.; Gupta, U. Impact of dendrimers on solubility of hydrophobic drug molecules. Front. Pharmacol. 2017, 8, 261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, J.; Jain, K.; Mehra, N.K.; Jain, N.K. Dendrimers in anticancer drug delivery: Mechanism of interaction of drug and dendrimers. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1626–1634. [Google Scholar] [CrossRef] [PubMed]
- Caminade, A.M.; Turrin, C.O. Dendrimers for drug delivery. J. Mater. Chem. B 2014, 2, 4055–4066. [Google Scholar] [CrossRef]
- Sanyakamdhorn, S.; Agudelo, D.; Bekale, L.; Tajmir-Riahi, H.A. Targeted conjugation of breast anticancer drug tamoxifen and its metabolites with synthetic polymers. Colloids Surf. B Biointerfaces 2016, 145, 55–63. [Google Scholar] [CrossRef]
- Liu, Y.; Ng, Y.; Toh, M.R.; Chiu, G.N.C. Lipid-dendrimer hybrid nanosystem as a novel delivery system for paclitaxel to treat ovarian cancer. J. Control. Release 2015, 220, 438–446. [Google Scholar] [CrossRef]
- Van Dongen, M.A.; Rattan, R.; Silpe, J.; Dougherty, C.; Michmerhuizen, N.L.; Van Winkle, M.; Huang, B.; Choi, S.K.; Sinniah, K.; Orr, B.G.; et al. Poly(amidoamine) dendrimer-methotrexate conjugates: The mechanism of interaction with folate binding protein. Mol. Pharm. 2014, 11, 4049–4058. [Google Scholar] [CrossRef] [Green Version]
- Zolotarskaya, O.Y.; Xu, L.; Valerie, K.; Yang, H. Click synthesis of a polyamidoamine dendrimer-based camptothecin prodrug. RSC Adv. 2015, 5, 58600–58608. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Da Rocha, S.R.P. Poly(amidoamine) dendrimer-doxorubicin conjugates: In vitro characteristics and pseudosolution formulation in pressurized metered-dose inhalers. Mol. Pharm. 2016, 13, 1058–1072. [Google Scholar] [CrossRef] [PubMed]
- Kale, A.A.; Torchilin, V.P. Design, synthesis, and characterization of pH-sensitive PEG-PE conjugates for stimuli-sensitive pharmaceutical nanocarriers: The effect of substitutes at the hydrazone linkage on the pH stability of PEG-PE conjugates. Bioconjug. Chem. 2007, 18, 363–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nieznanski, K.; Nieznanska, H.; Surewicz, W.K.; Surewicz, K.; Bandyszewska, M. US20190092837A1-Prion Protein-Dendrimer Conjugates for Use in Treatment of Alzheimer Disease. U.S. Patents Application No. 16/300,214, 28 March 2019. [Google Scholar]
- Zhang, L.; Zhu, S.; Qian, L.; Pei, Y.; Qiu, Y.; Jiang, Y. RGD-modified PEG-PAMAM-DOX conjugates: In vitro and in vivo studies for glioma. Eur. J. Pharm. Biopharm. 2011, 79, 232–240. [Google Scholar] [CrossRef]
- Satsangi, A.; Roy, S.S.; Satsangi, R.K.; Vadlamudi, R.K.; Ong, J.L. Design of a paclitaxel prodrug conjugate for active targeting of an enzyme upregulated in breast cancer cells. Mol. Pharm. 2014, 11, 1906–1918. [Google Scholar] [CrossRef]
- Dichwalkar, T.; Patel, S.; Bapat, S.; Pancholi, P.; Jasani, N.; Desai, B.; Yellepeddi, V.K.; Sehdev, V. Omega-3 fatty acid grafted PAMAM-paclitaxel conjugate exhibits enhanced anticancer activity in upper gastrointestinal cancer cells. Macromol. Biosci. 2017, 17, 1600457. [Google Scholar] [CrossRef]
- Yao, H.; Ma, J. Dendrimer-paclitaxel complexes for efficient treatment in ovarian cancer: Study on OVCAR-3 and HEK293T cells. Acta Biochim. Pol. 2018, 65, 219–225. [Google Scholar] [CrossRef]
- Kesharwani, P.; Iyer, A.K. Recent advances in dendrimer-based nanovectors for tumor-targeted drug and gene delivery. Drug Discov. Today 2015, 20, 536–547. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.S.; Nam, K.; Park, J.Y.; Kim, J.B.; Lee, J.K.; Park, J.S. Enhanced transfection efficiency of PAMAM dendrimer by surface modification with L-arginine. J. Control. Release 2004, 99, 445–456. [Google Scholar] [CrossRef]
- Ma, P.; Mumper, R.J. Paclitaxel nano-delivery systems: A comprehensive review. J. Nanomed. Nanotechnol. 2013, 4, 1000164. [Google Scholar] [CrossRef] [Green Version]
- Yellepeddi, V.K.; Kumar, A.; Maher, D.M.; Chauhan, S.C.; Vangara, K.K.; Palakurthi, S. Biotinylated PAMAM dendrimers for intracellular delivery of cisplatin to ovarian cancer: Role of SMVT. Anticancer Res. 2011, 31, 897–906. [Google Scholar] [PubMed]
- Tripodo, G.; Mandracchia, D.; Collina, S.; Rui, M.; Rossi, D. Medicinal chemistry new perspectives in cancer therapy: The biotin-antitumor molecule conjugates. Med. Chem. 2014, 1. [Google Scholar] [CrossRef] [Green Version]
- Bazak, R.; Houri, M.; El Achy, S.; Kamel, S.; Refaat, T. Cancer active targeting by nanoparticles: A comprehensive review of literature. J. Cancer Res. Clin. Oncol. 2015, 141, 769–784. [Google Scholar] [CrossRef] [Green Version]
- Kulhari, H.; Pooja, D.; Shrivastava, S.; Kuncha, M.; Naidu, V.G.M.; Bansal, V.; Sistla, R.; Adams, D.J. Trastuzumab-grafted PAMAM dendrimers for the selective delivery of anticancer drugs to HER2-positive breast cancer. Sci. Rep. 2016, 6, 23179. [Google Scholar] [CrossRef] [PubMed]
- Chung, A.; Cui, X.; Audeh, W.; Giuliano, A. Current status of anti-human epidermal growth factor receptor 2 therapies: Predicting and overcoming Herceptin resistance. Clin. Breast Cancer 2013, 13, 223–232. [Google Scholar] [CrossRef] [Green Version]
- Singh, M.K.; Kuncha, M.; Nayak, V.L.; Sarma, A.V.S.; Kumar, M.J.M.; Chauhan, A.S.; Sistla, R. An innovative in situ method of creating hybrid dendrimer nano-assembly: An efficient next generation dendritic platform for drug delivery. Nanomed. Nanotechnol. Biol. Med. 2019, 21, 102043. [Google Scholar] [CrossRef]
- Cheng, Y.; Xu, T. Dendrimers as versatile platform in drug delivery applications. Eur. J. Med. Chem. 2008, 43, 2291–2297. [Google Scholar] [CrossRef]
- Bawarski, W.E.; Chidlowsky, E.; Bharali, D.J.; Mousa, S.A. Emerging nanopharmaceuticals. Nanomed. Nanotechnol. Biol. Med. 2008, 4, 273–282. [Google Scholar] [CrossRef]
- Markowicz, M.; Szymánski, P.; Ciszewski, M.; Kłys, A.; Mikiciuk-Olasik, E. Evaluation of poly(amidoamine) dendrimers as potential carriers of iminodiacetic derivatives using solubility studies and 2D-NOESY NMR spectroscopy. J. Biol. Phys. 2012, 38, 637–656. [Google Scholar] [CrossRef] [Green Version]
- Karthikeyan, R.; Vijayarajkumar, P. PEGylated nanoarchitechture mediated solubility enhancement of tyrosine-kinase inhibitor. Inventi Rapid: Novel Excipients 2015, 2, 1–4. [Google Scholar]
- Hou, W.; Wei, P.; Kong, L.; Guo, R.; Wang, S.; Shi, X. Partially PEGylated dendrimer-entrapped gold nanoparticles: A promising nanoplatform for highly efficient DNA and siRNA delivery. J. Mater. Chem. B 2016, 4, 2933–2943. [Google Scholar] [CrossRef]
- Abedi-Gaballu, F.; Dehghan, G.; Ghaffari, M.; Yekta, R.; Abbaspour-Ravasjani, S.; Baradaran, B.; Dolatabadi, J.E.N.; Hamblin, M.R. PAMAM dendrimers as efficient drug and gene delivery nanosystems for cancer therapy. Appl. Mater. Today 2018, 12, 177–190. [Google Scholar] [CrossRef]
- Kesavan, A.; Ilaiyaraja, P.; Sofi Beaula, W.; Kumari, V.V.; Lal, J.S.; Arunkumar, C.; Anjana, G.; Srinivas, S.; Ramesh, A.; Rayala, S.K.; et al. Tumor targeting using polyamidoamine dendrimer-cisplatin nanoparticles functionalized with diglycolamic acid and herceptin. Eur. J. Pharm. Biopharm. 2015, 96, 255–263. [Google Scholar] [CrossRef]
- Mi, Y.; Zhao, J.; Feng, S.S. Targeted co-delivery of docetaxel, cisplatin and herceptin by vitamin E TPGS-cisplatin prodrug nanoparticles for multimodality treatment of cancer. J. Control. Release 2013, 169, 185–192. [Google Scholar] [CrossRef]
- Broome, A.-M. Advances in Cancer Research: Cancer Nanotechnology, 1st ed.; Elsevier Academic Press: Cambridge, MA, USA, 2018; Volume 139. [Google Scholar]
- Chitkara, D.; Ampama, M.; Mahato, R.I. Molecular Medicines for Cancer: Concepts and Applications of Nanotechn; CRC Press: Boca Raton, FL, USA, 2019; pp. 3–27. [Google Scholar]
- Esfand, R.; Tomalia, D.A. Poly(amidoamine) (PAMAM) dendrimers: From biomimicry to drug delivery and biomedical applications. Drug Discov. Today 2001, 6, 427–436. [Google Scholar] [CrossRef]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20. [Google Scholar] [CrossRef]
- Yadav, S.; Sharma, A.K.; Kumar, P. Nanoscale self-assembly for therapeutic delivery. Front. Bioeng. Biotechnol. 2020, 8, 127. [Google Scholar] [CrossRef]
- Le, P.N.; Nguyen, D.H.; Nguyen, C.K.; Tran, N.Q. Dendrimers for controlled release drug delivery. In Dendrimers for Drug Delivery; Apple Academic Press: Palm Bay, FL, USA, 2019; pp. 189–195. [Google Scholar]
- Kelley, S.O.; Stewart, K.M.; Mourtada, R. Development of novel peptides for mitochondrial drug delivery: Amino acids featuring delocalized lipophilic cations. Pharm. Res. 2011, 28, 2808–2819. [Google Scholar] [CrossRef]
- Patri, A.K.; Kukowska-Latallo, J.F.; Baker, J.R. Targeted drug delivery with dendrimers: Comparison of the release kinetics of covalently conjugated drug and non-covalent drug inclusion complex. Adv. Drug Deliv. Rev. 2005, 57, 2203–2214. [Google Scholar] [CrossRef]
- Nguyen, H.; Nguyen, N.H.; Tran, N.Q.; Nguyen, C.K. Improved method for preparing cisplatin-dendrimer nanocomplex and its behavior against NCI-H460 lung cancer cell. J. Nanosci. Nanotechnol. 2015, 15, 4106–4110. [Google Scholar] [CrossRef]
- Vu, M.T.; Bach, L.G.; Nguyen, D.C.; Ho, M.N.; Nguyen, N.H.; Tran, N.Q.; Nguyen, D.H.; Nguyen, C.K.; Hoang Thi, T.T. Modified carboxyl-terminated PAMAM dendrimers as great cytocompatible nano-based drug delivery system. Int. J. Mol. Sci. 2019, 20, 2016. [Google Scholar] [CrossRef] [Green Version]
- Salzano, G.; Navarro, G.; Trivedi, M.S.; De Rosa, G.; Torchilin, V.P. Multifunctional polymeric micelles co-loaded with anti-survivin siRNA and paclitaxel overcome drug resistance in an animal model of ovarian cancer. Mol. Cancer Ther. 2015, 14, 1075–1084. [Google Scholar] [CrossRef] [Green Version]
- Salzano, G.; Costa, D.F.; Sarisozen, C.; Luther, E.; Mattheolabakis, G.; Dhargalkar, P.P.; Torchilin, V.P. Mixed nanosized polymeric micelles as promoter of doxorubicin and miRNA-34a co-delivery triggered by dual stimuli in tumor tissue. Small 2016, 12, 4837–4848. [Google Scholar] [CrossRef]
- Garbuzenko, O.B.; Saad, M.; Pozharov, V.P.; Reuhl, K.R.; Mainelis, G.; Minko, T. Inhibition of lung tumor growth by complex pulmonary delivery of drugs with oligonucleotides as suppressors of cellular resistance. Proc. Natl. Acad. Sci. USA 2010, 107, 10737–10742. [Google Scholar] [CrossRef] [Green Version]
- Shcharbin, D.; Janaszewska, A.; Klajnert-Maculewicz, B.; Ziemba, B.; Dzmitruk, V.; Halets, I.; Loznikova, S.; Shcharbina, N.; Milowska, K.; Ionov, M.; et al. How to study dendrimers and dendriplexes III. Biodistribution, pharmacokinetics and toxicity in vivo. J. Control. Release 2014, 181, 40–52. [Google Scholar] [CrossRef]
- Haensler, J.; Szoka, F.C. Polyamidoamine cascade polymers mediate efficient transfection of cells in culture. Bioconjug. Chem. 1993, 4, 372–379. [Google Scholar] [CrossRef]
- Wang, K.; Hu, Q.; Zhu, W.; Zhao, M.; Ping, Y.; Tang, G. Structure-invertible nanoparticles for triggered co-delivery of nucleic acids and hydrophobic drugs for combination cancer therapy. Adv. Funct. Mater. 2015, 25, 3380–3392. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Wang, K.; Hu, Q.; Yao, Q.; Shen, Y.; Yu, G.; Tang, G. Targeted Co-delivery of PTX and TR3 siRNA by PTP Peptide modified dendrimer for the treatment of pancreatic cancer. Small 2017, 13, 1602697. [Google Scholar] [CrossRef]
- Caminade, A.-M. Phosphorus dendrimers as nanotools against cancers. Molecules 2020, 25, 3333. [Google Scholar] [CrossRef]
- Li, X.; Wu, X.; Yang, H.; Li, L.; Ye, Z.; Rao, Y. A nuclear targeted Dox-aptamer loaded liposome delivery platform for the circumvention of drug resistance in breast cancer. Biomed. Pharmacother. 2019, 117, 109072. [Google Scholar] [CrossRef]
- Gu, Y.; Guo, Y.; Wang, C.; Xu, J.; Wu, J.; Kirk, T.B.; Ma, D.; Xue, W. A polyamidoamne dendrimer functionalized graphene oxide for DOX and MMP-9 shRNA plasmid co-delivery. Mater. Sci. Eng. C 2017, 70, 572–585. [Google Scholar] [CrossRef]
- Kannan, R.M.; Nance, E.; Kannan, S.; Tomalia, D.A. Emerging concepts in dendrimer-based nanomedicine: From design principles to clinical applications. J. Intern. Med. 2014, 276, 579–617. [Google Scholar] [CrossRef]
- Inoue, K. Functional dendrimers, hyperbranched and star polymers. Prog. Polym. Sci. 2000, 25, 453–571. [Google Scholar] [CrossRef]
- Tack, F.; Bakker, A.; Maes, S.; Dekeyser, N.; Bruining, M.; Elissen-Roman, C.; Janicot, M.; Brewster, M.; Janssen, H.M.; De Waal, B.F.M.; et al. Modified poly(propylene imine) dendrimers as effective transfection agents for catalytic DNA enzymes (DNAzymes). J. Drug Target. 2006, 14, 69–86. [Google Scholar] [CrossRef]
- Kesharwani, P.; Tekade, R.K.; Jain, N.K. Generation dependent safety and efficacy of folic acid conjugated dendrimer based anticancer drug formulations. Pharm. Res. 2015, 32, 1438–1450. [Google Scholar] [CrossRef]
- Jain, N.K.; Tare, M.S.; Mishra, V.; Tripathi, P.K. The development, characterization and in vivo anti-ovarian cancer activity of poly(propylene imine) (PPI)-antibody conjugates containing encapsulated paclitaxel. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 207–218. [Google Scholar] [CrossRef]
- Gorzkiewicz, M.; Klajnert-Maculewicz, B. Dendrimers as Nanocarriers for Anticancer Drugs; Apple Academic Press: Palm Bay, FL, USA, 2019; pp. 327–362. [Google Scholar]
- Tekade, R.K.; Dutta, T.; Tyagi, A.; Bharti, A.C.; Das, B.C.; Jain, N.K. Surface-engineered dendrimers for dual drug delivery: A receptor up-regulation and enhanced cancer targeting strategy. J. Drug Target. 2008, 16, 758–772. [Google Scholar] [CrossRef]
- Qi, R.; Majoros, I.; Misra, A.C.; Koch, A.E.; Campbell, P.; Marotte, H.; Bergin, I.L.; Cao, Z.; Goonewardena, S.; Morry, J.; et al. Folate receptor-targeted dendrimer-methotrexate conjugate for inflammatory arthritis. J. Biomed. Nanotechnol. 2015, 11, 1431–1441. [Google Scholar] [CrossRef]
- Mintzer, M.A.; Grinstaff, M.W. Biomedical applications of dendrimers: A tutorial. Chem. Soc. Rev. 2011, 40, 173–190. [Google Scholar] [CrossRef]
- Al-Jamal, K.T.; Al-Jamal, W.T.; Wang, J.T.W.; Rubio, N.; Buddle, J.; Gathercole, D.; Zloh, M.; Kostarelos, K. Cationic poly-L-lysine dendrimer complexes doxorubicin and delays tumor growth in vitro and in vivo. ACS Nano 2013, 7, 1905–1917. [Google Scholar] [CrossRef]
- Wu, J.; Huang, W.; He, Z. Dendrimers as carriers for siRNA delivery and gene silencing: A review. Sci. World J. 2013, 2013. [Google Scholar] [CrossRef] [Green Version]
- Bugno, J.; Hsu, H.J.; Pearson, R.M.; Noh, H.; Hong, S. Size and surface charge of engineered poly(amidoamine) dendrimers modulate tumor accumulation and penetration: A model study using multicellular tumor spheroids. Mol. Pharm. 2016, 13, 2155–2163. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Z.; Xu, X.; Li, Y.; Li, Y.; Jian, Y.; Gu, Z. Bioinspired therapeutic dendrimers as efficient peptide drugs based on supramolecular interactions for tumor inhibition. Angew. Chem. 2015, 54, 4289–4294. [Google Scholar] [CrossRef]
- Ahmed, S.; Vepuri, S.B.; Kalhapure, R.S.; Govender, T. Interactions of dendrimers with biological drug targets: Reality or mystery-a gap in drug delivery and development research. Biomater. Sci. 2016, 4, 1032–1050. [Google Scholar] [CrossRef]
- European Medicines Agency. Guideline on Excipients in the Dossier for Application for Marketing Authorisation of a Medicinal Product; EMA: London, UK, 2007. [Google Scholar]
- Dzmitruk, V.; Szulc, A.; Shcharbin, D.; Janaszewska, A.; Shcharbina, N.; Lazniewska, J.; Novopashina, D.; Buyanova, M.; Ionov, M.; Klajnert-Maculewicz, B.; et al. Anticancer siRNA cocktails as a novel tool to treat cancer cells. Part (B). Efficiency of pharmacological action. Int. J. Pharm. 2015, 485, 288–294. [Google Scholar] [CrossRef]
- Katir, N.; Majoral, J.P.; El Kadib, A.; Caminade, A.-M.; Bousmina, M. Molecular and macromolecular engineering with viologens as building blocks: Rational design of phosphorus-viologen dendritic structures. Eur. J. Org. Chem. 2012, 2012, 269–273. [Google Scholar] [CrossRef]
- Ciepluch, K.; Katir, N.; El Kadib, A.; Felczak, A.; Zawadzka, K.; Weber, M.; Klajnert, B.; Lisowska, K.; Caminade, A.M.; Bousmina, M.; et al. Biological properties of new viologen-phosphorus dendrimers. Mol. Pharm. 2012, 9, 448–457. [Google Scholar] [CrossRef]
- Lazniewska, J.; Janaszewska, A.; Miłowska, K.; Caminade, A.-M.; Mignani, S.; Katir, N.; Kadib, A.; Bryszewska, M.; Majoral, J.-P.; Gabryelak, T.; et al. Promising low-toxicity of viologen-phosphorus dendrimers against embryonic mouse hippocampal cells. Molecules 2013, 18, 12222–12240. [Google Scholar] [CrossRef]
- Liu, B.; Huang, X.P.; Hu, Y.L.; Chen, T.T.; Peng, B.Y.; Gao, N.N.; Jin, Z.C.; Jia, T.L.; Zhang, N.; Wang, Z.L.; et al. Ethacrynic acid improves the antitumor effects of irreversible epidermal growth factor receptor tyrosine kinase inhibitors in breast cancer. Oncotarget 2016, 7, 58038–58050. [Google Scholar] [CrossRef]
- Al-Dali, A.M.; Weiher, H.; Schmidt-Wolf, I.G.H. Utilizing ethacrynic acid and ciclopirox olamine in liver cancer. Oncol. Lett. 2018, 16, 6854–6860. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.J.; Song, H.; Yoon, Y.J.; Park, S.J.; Kim, S.Y.; Cho Han, D.; Kwon, B.M. Ethacrynic acid inhibits STAT3 activity through the modulation of SHP2 and PTP1B tyrosine phosphatases in DU145 prostate carcinoma cells. Biochem. Pharmacol. 2020, 175, 113920. [Google Scholar] [CrossRef] [PubMed]
- El Brahmi, N.; Mignani, S.M.; Caron, J.; El Kazzouli, S.; Bousmina, M.M.; Caminade, A.M.; Cresteil, T.; Majoral, J.P. Investigations on dendrimer space reveal solid and liquid tumor growth-inhibition by original phosphorus-based dendrimers and the corresponding monomers and dendrons with ethacrynic acid motifs. Nanoscale 2015, 7, 3915–3922. [Google Scholar] [CrossRef] [PubMed]
- Mignani, S.; El Brahmi, N.; El Kazzouli, S.; Eloy, L.; Courilleau, D.; Caron, J.; Bousmina, M.M.; Caminade, A.M.; Cresteil, T.; Majoral, J.P. A novel class of ethacrynic acid derivatives as promising drug-like potent generation of anticancer agents with established mechanism of action. Eur. J. Med. Chem. 2016, 122, 656–673. [Google Scholar] [CrossRef]
- Servin, P.; Laurent, R.; Tristany, M.; Romerosa, A.; Peruzzini, M.; Garcia-Maroto, F.; Majoral, J.P.; Caminade, A.M. Dual properties of water-soluble Ru-PTA complexes of dendrimers: Catalysis and interaction with DNA. Inorg. Chim. Acta 2018, 470, 106–112. [Google Scholar] [CrossRef] [Green Version]
- El Brahmi, N.; El Kazzouli, S.; Mignani, S.M.; Essassi, E.M.; Aubert, G.; Laurent, R.; Caminade, A.M.; Bousmina, M.M.; Cresteil, T.; Majoral, J.P. Original multivalent copper(II)-conjugated phosphorus dendrimers and corresponding mononuclear copper(II) complexes with antitumoral activities. Mol. Pharm. 2013, 10, 1459–1464. [Google Scholar] [CrossRef]
- Mignani, S.M.; El Brahmi, N.; El Kazzouli, S.; Laurent, R.; Ladeira, S.; Caminade, A.M.; Pedziwiatr-Werbicka, E.; Szewczyk, E.M.; Bryszewska, M.; Bousmina, M.M.; et al. Original multivalent Gold(III) and dual Gold(III)-Copper(II) conjugated phosphorus dendrimers as potent antitumoral and antimicrobial agents. Mol. Pharm. 2017, 14, 4087–4097. [Google Scholar] [CrossRef]
- Vanerio, N.; Stijnen, M.; de Mol, B.A.J.M.; Kock, L.M. Biomedical Applications of photo- and sono-activated rose bengal: A review. Photobiomodul. Photomed. Laser Surg. 2019, 37, 383–394. [Google Scholar] [CrossRef] [Green Version]
- Dabrzalska, M.; Janaszewska, A.; Zablocka, M.; Mignani, S.; Majoral, J.P.; Klajnert-Maculewicz, B. Cationic phosphorus dendrimer enhances photodynamic activity of rose bengal against basal cell carcinoma cell lines. Mol. Pharm. 2017, 14, 1821–1830. [Google Scholar] [CrossRef]
- Kesharwani, P.; Jain, K.; Jain, N.K. Dendrimer as nanocarrier for drug delivery. Prog. Polym. Sci. 2014, 39, 268–307. [Google Scholar] [CrossRef]
- Lipinski, C.A. Poor aqueous solubility—An industry wide problem in drug discovery. Am. Pharm. Res. 2002, 19, 1894–1900. [Google Scholar]
- Leuner, C.; Dressman, J. Improving drug solubility for oral delivery using solid dispersions. Eur. J. Pharm. Biopharm. 2000, 50, 47–60. [Google Scholar] [CrossRef]
- Yiyun, C.; Jeipin, Y. Solubilization of non-steroidal anti-inflammatory drugs in the presence of tween series surfactants. Phys. Chem. Liq. 2006, 44, 249–256. [Google Scholar] [CrossRef]
- Yiyun, C.; Tongwen, X. Dendrimers as potential drug carriers. Part I. Solubilization of non-steroidal anti-inflammatory drugs in the presence of polyamidoamine dendrimers. Eur. J. Med. Chem. 2005, 40, 1188–1192. [Google Scholar] [CrossRef]
- Ullah, I.; Baloch, M.K.; Durrani, G.F. Solubility of nonsteroidal anti-inflammatory drugs (NSAIDs) in aqueous solutions of non-ionic surfactants. J. Solut. Chem. 2011, 40, 1341–1348. [Google Scholar] [CrossRef]
- Ihre, H.R.; de Jesús, O.L.P.; Szoka, F.C.; Fréchet, J.M.J. Polyester dendritic systems for drug delivery applications: Design, synthesis, and characterization. Bioconjug. Chem. 2002, 13, 443–452. [Google Scholar] [CrossRef]
- Markowicz-Piasecka, M.; Mikiciuk-Olasik, E. Nanobiomaterials in Drug Delivery, 1st ed.; Elsevier: Norwich, NY, USA, 2016; Volume 9, pp. 39–74. [Google Scholar]
- Beezer, A.E.; King, A.S.H.; Martin, I.K.; Mitchel, J.C.; Twyman, L.J.; Wain, C.F. Dendrimers as potential drug carriers; encapsulation of acidic hydrophobes within water soluble PAMAM derivatives. Tetrahedron 2003, 59, 3873–3880. [Google Scholar] [CrossRef]
- Durocher, I.; Girard, D. In vivo proinflammatory activity of generations 0–3 (G0–G3) polyamidoamine (PAMAM) nanoparticles. Inflamm. Res. 2016, 65, 745–755. [Google Scholar] [CrossRef]
- Scorciapino, M.; Serra, I.; Manzo, G.; Rinaldi, A. Antimicrobial dendrimeric peptides: Structure, activity and new therapeutic applications. Int. J. Mol. Sci. 2017, 18, 542. [Google Scholar] [CrossRef] [Green Version]
- Irvine, J.; Afrose, A.; Islam, N. Formulation and delivery strategies of ibuprofen: Challenges and opportunities. Drug Dev. Ind. Pharm. 2018, 44, 173–183. [Google Scholar] [CrossRef]
- Garavito, R.M.; Malkowski, M.G.; DeWitt, D.L. The structures of prostaglandin endoperoxide H synthases-1 and -2. Prostaglandins Lipid Mediat. 2002, 68–69, 129–152. [Google Scholar] [CrossRef]
- Kawai, S.; Kojima, F.; Kusunoki, N. Recent advances in nonsteroidal anti-inflammatory drugs. Allergol. Int. 2005, 54, 209–215. [Google Scholar] [CrossRef] [Green Version]
- Kean, W.F.; Buchanan, W.W. The use of NSAIDs in rheumatic disorders 2005: A global perspective. Inflammopharmacology 2005, 13, 343–370. [Google Scholar] [CrossRef] [PubMed]
- Bonelli, P.; Tuccillo, F.M.; Calemma, R.; Pezzetti, F.; Borrelli, A.; Martinelli, R.; De Rosa, A.; Esposito, D.; Palaia, R.; Castello, G. Changes in the gene expression profile of gastric cancer cells in response to ibuprofen: A gene pathway analysis. Pharm. J. 2011, 11, 412–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurtoglu, Y.E.; Mishra, M.K.; Kannan, S.; Kannan, R.M. Drug release characteristics of PAMAM dendrimer-drug conjugates with different linkers. Int. J. Pharm. 2010, 384, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Kolhe, P.; Khandare, J.; Pillai, O.; Kannan, S.; Lieh-Lai, M.; Kannan, R.M. Preparation, cellular transport, and activity of polyamidoamine-based dendritic nanodevices with a high drug payload. Biomaterials 2006, 27, 660–669. [Google Scholar] [CrossRef]
- Avti, P.K.; Kakkar, A. Dendrimers as anti-inflammatory agents. Braz. J. Pharm. Sci. 2013, 49, 57–65. [Google Scholar] [CrossRef] [Green Version]
- Kolhe, P.; Misra, E.; Kannan, R.M.; Kannan, S.; Lieh-Lai, M. Drug complexation, in vitro release and cellular entry of dendrimers and hyperbranched polymers. Int. J. Pharm. 2003, 259, 143–160. [Google Scholar] [CrossRef] [Green Version]
- Koç, F.E.; Şenel, M. Solubility enhancement of Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) using polypolypropylene oxide core PAMAM dendrimers. Int. J. Pharm. 2013, 451, 18–22. [Google Scholar] [CrossRef]
- Ohshima, H.; Makino, K. Colloid and Interface Science in Pharmaceutical Research and Development; Elsevier Inc.: Amsterdam, The Netherlands, 2014; ISBN 9780444626080. [Google Scholar]
- Weledji, E.P.; Weledji, E.K.; Assob, J.C.; Nsagha, D.S. Pros, cons and future of antibiotics. New Horiz. Transl. Med. 2017, 4, 9–14. [Google Scholar] [CrossRef]
- Tsafnat, G.; Copty, J.; Partridge, S.R. RAC: Repository of Antibiotic resistance Cassettes. Database 2011, 2011, bar054. [Google Scholar] [CrossRef] [Green Version]
- Spellberg, B.; Bartlett, J.G.; Gilbert, D.N. The future of antibiotics and resistance. N. Engl. J. Med. 2013, 368, 299–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walsh, F. The multiple roles of antibiotics and antibiotic resistance in nature. Front. Microbiol. 2013, 4, 255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Partridge, S.R.; Tsafnat, G. Automated annotation of mobile antibiotic resistance in Gram-negative bacteria: The Multiple Antibiotic Resistance Annotator (MARA) and database. J. Antimicrob. Chemother. 2018, 73, 883–890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.Y.; Lee, W.S.; Fung, C.P.; Cheng, N.C.; Liu, C.L.; Yang, S.P.; Chen, S.L. Comparative study of teicoplanin vs vancomycin for the treatment of methicillin-resistant Staphylococcus aureus bacteraemia. Clin. Drug Investig. 1996, 12, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Casadevall, A.; Pirofski, L.A. The damage-response framework of microbial pathogenesis. Nat. Rev. Microbiol. 2003, 1, 17–24. [Google Scholar] [CrossRef]
- Petrosillo, N. Infections: The emergency of the new millennium. In Nuclear Medicine in Infectious Diseases; Springer: Cham, Switzerland, 2020; pp. 1–8. [Google Scholar]
- Srividya, N.; Ghoora, M.D.; Padmanabh, P.R. Antimicrobial nanotechnology: Research implications and prospects in food safety. In Food Preservation; Elsevier: Cambridge, MA, USA, 2017; pp. 125–165. [Google Scholar]
- Karthikeyan, R.; Koushik, O.S.; Kumar, P.V. Dendrimeric architecture for effective antimicrobial therapy in: Dendrimers for drug delivery. In Dendrimers for Controlled Release Drug Delivery; Apple Academic Press Inc.: Palm Bay, FL, USA, 2019; pp. 375–405. [Google Scholar]
- Authimoolam, S.; Dziubla, T. Biopolymeric mucin and synthetic polymer analogs: Their structure, function and role in biomedical applications. Polymers 2016, 8, 71. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Qu, H.; Ma, M.; Xu, Z.; Xu, P.; Fang, Y.; Xu, T. Polyamidoamine (PAMAM) dendrimers as biocompatible carriers of quinolone antimicrobials: An in vitro study. Eur. J. Med. Chem. 2007, 42, 1032–1038. [Google Scholar] [CrossRef]
- Kuwahara, K.; Kitazawa, T.; Kitagaki, H.; Tsukamoto, T.; Kikuchi, M. Nadifloxacin, an antiacne quinolone antimicrobial, inhibits the production of proinflammatory cytokines by human peripheral blood mononuclear cells and normal human keratinocytes. J. Dermatol. Sci. 2005, 38, 47–55. [Google Scholar] [CrossRef]
- Svenningsen, S.W.; Frederiksen, R.F.; Counil, C.; Ficker, M.; Leisner, J.J.; Christensen, J.B. Synthesis and Antimicrobial Properties of a Ciprofloxacin and PAMAM-dendrimer Conjugate. Molecules 2020, 25, 1389. [Google Scholar] [CrossRef] [Green Version]
- Wrońska, N.; Majoral, J.P.; Appelhans, D.; Bryszewska, M.; Lisowska, K. Synergistic effects of anionic/cationic dendrimers and levofloxacin on antibacterial activities. Molecules 2019, 24, 2894. [Google Scholar] [CrossRef] [Green Version]
- Mulani, M.S.; Kamble, E.E.; Kumkar, S.N.; Tawre, M.S.; Pardesi, K.R. Emerging strategies to combat ESKAPE pathogens in the era of antimicrobial resistance: A review. Front. Microbiol. 2019, 10, 539. [Google Scholar] [CrossRef] [PubMed]
- Boucher, H.W.; Talbot, G.H.; Bradley, J.S.; Edwards, J.E.; Gilbert, D.; Rice, L.B.; Scheld, M.; Spellberg, B.; Bartlett, J. Bad bugs, no drugs: No ESKAPE! An update from the infectious diseases society of America. Clin. Infect. Dis. 2009, 48, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Kamaruzzaman, N.F.; Tan, L.P.; Hamdan, R.H.; Choong, S.S.; Wong, W.K.; Gibson, A.J.; Chivu, A.; Pina, M.D.F. Antimicrobial polymers: The potential replacement of existing antibiotics? Int. J. Mol. Sci. 2019, 20, 2747. [Google Scholar] [CrossRef] [Green Version]
- Lindmeier, C. High Levels of Antibiotic Resistance Found Worldwide, New Data Shows; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Hong, L.; Luo, S.-H.; Yu, C.-H.; Xie, Y.; Xia, M.-Y.; Chen, G.-Y.; Peng, Q. Functional nanomaterials and their potential applications in antibacterial therapy. Pharm. Nanotechnol. 2019, 7, 129–146. [Google Scholar] [CrossRef]
- Shrestha, A.; Kishen, A. Antibacterial nanoparticles in endodontics: A review. J. Endod. 2016, 42, 1417–1426. [Google Scholar] [CrossRef]
- Niemirowicz, K.; Durnaś, B.; Piktel, E.; Bucki, R. Development of antifungal therapies using nanomaterials. Nanomedicine 2017, 12, 1891–1905. [Google Scholar] [CrossRef]
- Zhu, G.Y.; Lu, B.Y.; Zhang, T.X.; Zhang, T.; Zhang, C.L.; Li, Y.; Peng, Q. Antibiofilm effect of drug-free and cationic poly(D,L-lactide-co-glycolide) nanoparticles via nano-bacteria interactions. Nanomedicine 2018, 13, 1093–1106. [Google Scholar] [CrossRef]
- Winnicka, K.; Wroblewska, M.; Wieczorek, P.; Sacha, P.; Tryniszewska, E. The effect of PAMAM dendrimers on the antibacterial activity of antibiotics with different water solubility. Molecules 2013, 18, 8607–8617. [Google Scholar] [CrossRef] [Green Version]
- Kawano, Y.; Jordan, O.; Hanawa, T.; Borchard, G.; Patrulea, V. Are antimicrobial peptide dendrimers an escape from ESKAPE? Adv. Wound Care 2020, 9, 378–395. [Google Scholar] [CrossRef]
- García-Gallego, S.; Franci, G.; Falanga, A.; Gómez, R.; Folliero, V.; Galdiero, S.; de la Mata, F.; Galdiero, M. Function Oriented Molecular Design: Dendrimers as Novel Antimicrobials. Molecules 2017, 22, 1581. [Google Scholar] [CrossRef]
- McNerny, D.Q.; Leroueil, P.R.; Baker, J.R. Understanding specific and nonspecific toxicities: A requirement for the development of dendrimer-based pharmaceuticals. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2010, 2, 249–259. [Google Scholar] [CrossRef] [Green Version]
- Lopez, A.I.; Reins, R.Y.; McDermott, A.M.; Trautner, B.W.; Cai, C. Antibacterial activity and cytotoxicity of PEGylated poly(amidoamine) dendrimers. Mol. Biosyst. 2009, 5, 1148–1156. [Google Scholar] [CrossRef]
- Ortega, P.; Copa-Patiño, J.L.; Muñoz-Fernandez, M.A.; Soliveri, J.; Gomez, R.; De La Mata, F.J. Amine and ammonium functionalization of chloromethylsilane-ended dendrimers. Antimicrobial activity studies. Org. Biomol. Chem. 2008, 6, 3264–3269. [Google Scholar] [CrossRef]
- Fallah, F.; Zargar, M.; Yousefi, M.; Alam, A.N. Synthesis of the erythromycin-conjugated nanodendrimer and its antibacterial activity. Eur. J. Pharm. Sci. 2018, 123, 321–326. [Google Scholar] [CrossRef]
- Xue, X.; Chen, X.; Mao, X.; Hou, Z.; Zhou, Y.; Bai, H.; Meng, J.; Da, F.; Sang, G.; Wang, Y.; et al. Amino-terminated generation 2 poly(amidoamine) dendrimer as a potential broad-spectrum, nonresistance-inducing antibacterial agent. AAPS J. 2013, 15, 132–142. [Google Scholar] [CrossRef] [Green Version]
- Calabretta, M.K.; Kumar, A.; McDermott, A.M.; Cai, C. Antibacterial activities of poly(amidoamine) dendrimers terminated with amino and poly(ethylene glycol) groups. Biomacromolecules 2007, 8, 1807–1811. [Google Scholar] [CrossRef] [Green Version]
- Wróblewska, M.; Winnicka, K. The effect of cationic polyamidoamine dendrimers on physicochemical characteristics of hydrogels with erythromycin. Int. J. Mol. Sci. 2015, 16, 20277–20289. [Google Scholar] [CrossRef] [Green Version]
- Lewis, K. Antibiotics: Recover the lost art of drug discovery. Nature 2012, 485, 439–440. [Google Scholar] [CrossRef]
- Brownlie, J.; Peckham, C.; Waage, J.; Woolhouse, M.; Lyall, C.; Meagher, L.; Tait, J.; Baylis, M.; Nicoll, A. Infectious Diseases: Preparing for the Future—Future Threats; Office of Science and Innovation: London, UK, 2006. [Google Scholar]
- Mhlwatika, Z.; Aderibigbe, B. Application of dendrimers for the treatment of infectious diseases. Molecules 2018, 23, 2205. [Google Scholar] [CrossRef] [Green Version]
- Vacas-Córdoba, E.; Maly, M.; De la Mata, F.J.; Gómez, R.; Pion, M.; Muñoz-Fernández, M.Á. Antiviral mechanism of polyanionic carbosilane dendrimers against HIV-I. Int. J. Nanomed. 2016, 11, 1281–1294. [Google Scholar] [CrossRef] [Green Version]
- Relaño-Rodríguez, I.; Juárez-Sánchez, R.; Pavicic, C.; Muñoz, E.; Muñoz-Fernández, M.Á. Polyanionic carbosilane dendrimers as a new adjuvant in combination with latency reversal agents for HIV treatment. J. Nanobiotechnol. 2019, 17, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasines, B.; Sánchez-Nieves, J.; Maiolo, M.; Maly, M.; Chonco, L.; Jiménez, J.L.; Muñoz-Fernández, M.Á.; De La Mata, F.J.; Gómez, R. Synthesis, structure and molecular modelling of anionic carbosilane dendrimers. Dalton Trans. 2012, 41, 12733–12748. [Google Scholar] [CrossRef] [PubMed]
- Arnáiz, E.; Vacas-Córdoba, E.; Galán, M.; Pion, M.; Gómez, R.; Muñoz-Fernández, M.Á.; de la Mata, F.J. Synthesis of anionic carbosilane dendrimers via “click chemistry” and their antiviral properties against HIV. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 1099–1112. [Google Scholar] [CrossRef]
- Sepúlveda-Crespo, D.; Gómez, R.; De La Mata, F.J.; Jiménez, J.L.; Muñoz-Fernández, M.Á. Polyanionic carbosilane dendrimer-conjugated antiviral drugs as efficient microbicides: Recent trends and developments in HIV treatment/therapy. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1481–1498. [Google Scholar] [CrossRef]
- Sepúlveda-Crespo, D.; Sánchez-Rodríguez, J.; Serramía, M.J.; Gómez, R.; De La Mata, F.J.; Jiménez, J.L.; Muñoz-Fernández, M.Á. Triple combination of carbosilane dendrimers, tenofovir and maraviroc as potential microbicide to prevent HIV-1 sexual transmission. Nanomedicine 2015, 10, 899–914. [Google Scholar] [CrossRef]
- Günther, S.C.; Maier, J.D.; Vetter, J.; Podvalnyy, N.; Khanzhin, N.; Hennet, T.; Stertz, S. Antiviral potential of 3′-sialyllactose- and 6′-sialyllactose-conjugated dendritic polymers against human and avian influenza viruses. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Sandoval-Yañez, C.; Rodriguez, C.C. Dendrimers: Amazing platforms for bioactive molecule delivery systems. Materials 2020, 13, 570. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Gu, C.; Cabigas, E.B.; Pendergrass, K.D.; Brown, M.E.; Luo, Y.; Davis, M.E. Functionalized dendrimer-based delivery of angiotensin type 1 receptor siRNA for preserving cardiac function following infarction. Biomaterials 2013, 34, 3729–3736. [Google Scholar] [CrossRef] [Green Version]
- Singh, M.K.; Pooja, D.; Kulhari, H.; Jain, S.K.; Sistla, R.; Chauhan, A.S. Poly (amidoamine) dendrimer-mediated hybrid formulation for combination therapy of ramipril and hydrochlorothiazide. Eur. J. Pharm. Sci. 2017, 96, 84–92. [Google Scholar] [CrossRef]
- Ertürk, A.S.; Gürbüz, M.U.; Tülü, M. The effect of PAMAM dendrimer concentration, generation size and surface functional group on the aqueous solubility of candesartan cilexetil. Pharm. Dev. Technol. 2017, 22, 111–121. [Google Scholar] [CrossRef]
- Kulhari, H.; Pooja, D.; Prajapati, S.K.; Chauhan, A.S. Performance evaluation of PAMAM dendrimer based simvastatin formulations. Int. J. Pharm. 2011, 405, 203–209. [Google Scholar] [CrossRef]
- Oberdörster, G.; Maynard, A.; Donaldson, K.; Castranova, V.; Fitzpatrick, J.; Ausman, K.; Carter, J.; Karn, B.; Kreyling, W.; Lai, D.; et al. Principles for characterizing the potential human health effects from exposure to nanomaterials: Elements of a screening strategy. Part. Fibre Toxicol. 2005, 2, 1–35. [Google Scholar] [CrossRef]
- Mostafavi, E.; Soltantabar, P.; Webster, T.J. Nanotechnology and picotechnology. In Biomaterials in Translational Medicine; Elsevier Inc.: London, UK, 2019; pp. 191–212. [Google Scholar]
- Pillai, G. Nanotechnology toward treating cancer. In Applications of Targeted Nano Drugs and Delivery Systems; Elsevier: London, UK, 2019; pp. 221–256. [Google Scholar]
- Garriguea, P.; Tang, J.; Ding, L.; Bouhlel, A.; Tintaru, A.; Laurini, E.; Huang, Y.; Lyu, Z.; Zhang, M.; Fernandez, S.; et al. Self-assembling supramolecular dendrimer nanosystem for PET imaging of tumors. Proc. Natl. Acad. Sci. USA 2018, 115, 11454–11459. [Google Scholar] [CrossRef] [Green Version]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control. Release 2000, 65, 271–284. [Google Scholar] [CrossRef]
- Chow, E.K.H.; Ho, D. Cancer nanomedicine: From drug delivery to imaging. Sci. Transl. Med. 2013, 5, 216rv4. [Google Scholar] [CrossRef]
- Li, C. A targeted approach to cancer imaging and therapy. Nat. Mater. 2014, 13, 110–115. [Google Scholar] [CrossRef] [Green Version]
- Percec, V.; Wilson, D.A.; Leowanawat, P.; Wilson, C.J.; Hughes, A.D.; Kaucher, M.S.; Hammer, D.A.; Levine, D.H.; Kim, A.J.; Bates, F.S.; et al. Self-assembly of Janus dendrimers into uniform dendrimersomes and other complex architectures. Science 2010, 328, 1009–1014. [Google Scholar] [CrossRef]
- Wei, T.; Chen, C.; Liu, J.; Liu, C.; Posocco, P.; Liu, X.; Cheng, Q.; Huo, S.; Liang, Z.; Fermeglia, M.; et al. Anticancer drug nanomicelles formed by self-assembling amphiphilic dendrimer to combat cancer drug resistance. Proc. Natl. Acad. Sci. USA 2015, 112, 2978–2983. [Google Scholar] [CrossRef] [Green Version]
- Sherman, S.E.; Xiao, Q.; Percec, V. Mimicking complex biological membranes and their programmable glycan ligands with dendrimersomes and glycodendrimersomes. Chem. Rev. 2017, 117, 6538–6631. [Google Scholar] [CrossRef]
- Cutler, C.S.; Hennkens, H.M.; Sisay, N.; Huclier-Markai, S.; Jurisson, S.S. Radiometals for combined imaging and therapy. Chem. Rev. 2013, 113, 858–883. [Google Scholar] [CrossRef]
- Markowicz-Piasecka, M.; Sikora, J.; Szymanski, P.; Kozak, O.; Studniarek, M.; Mikiciuk-Olasik, E. PAMAM dendrimers as potential carriers of gadolinium complexes of iminodiacetic acid derivatives for magnetic resonance imaging. J. Nanomater. 2015. [Google Scholar] [CrossRef]
- Szymański, P.; Markowicz, M.; Mikiciuk-Olasik, E. Nanotechnology in pharmaceutical and biomedical applications: Dendrimers. Nano 2011, 6, 509–539. [Google Scholar] [CrossRef]
- Caravan, P. Strategies for increasing the sensitivity of gadolinium based MRI contrast agents. Chem. Soc. Rev. 2006, 35, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Dadiani, M.; Furman-Haran, E.; Degani, H. The application of NMR in tumor angiogenesis research. Prog. Nucl. Magn. Reson. Spectrosc. 2006, 49, 27–44. [Google Scholar] [CrossRef]
- Longmire, M.R.; Ogawa, M.; Choyke, P.L.; Kobayashi, H. Dendrimers as high relaxivity MR contrast agents. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2014, 6, 155–162. [Google Scholar] [CrossRef]
- McMahon, M.T.; Bulte, J.W.M. Two decades of dendrimers as versatile MRI agents: A tale with and without metals. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2018, 10, e1496. [Google Scholar] [CrossRef]
- Wu, L.P.; Ficker, M.; Christensen, J.B.; Trohopoulos, P.N.; Moghimi, S.M. Dendrimers in medicine: Therapeutic concepts and pharmaceutical challenges. Bioconjug. Chem. 2015, 26, 1198–1211. [Google Scholar] [CrossRef]
- Campbell-Washburn, A.E.; Ramasawmy, R.; Restivo, M.C.; Bhattacharya, I.; Basar, B.; Herzka, D.A.; Hansen, M.S.; Rogers, T.; Patricia Bandettini, W.; McGuirt, D.R.; et al. Opportunities in interventional and diagnostic imaging by using high-performance low-field-strength MRI. Radiology 2019, 293, 384–393. [Google Scholar] [CrossRef]
- Rai, D.B.; Gupta, N.; Pooja, D.; Kulhari, H. Chapter 13. Dendrimers for diagnostic applications. In Pharmaceutical Applications of Dendrimers; Chauhan, A., Kulhari, H., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 291–324. [Google Scholar]
- Sk, U.H.; Kojima, C. Dendrimers for theranostic applications. Biomol. Concepts 2015, 6, 205–217. [Google Scholar] [CrossRef]
- Winalski, C.S.; Shortkroff, S.; Mulkern, R.V.; Schneider, E.; Rosen, G.M. Magnetic resonance relaxivity of dendrimer-linked nitroxides. Magn. Reson. Med. 2002, 48, 965–972. [Google Scholar] [CrossRef]
- Maliakal, A.J.; Turro, N.J.; Bosman, A.W.; Cornel, J.; Meijer, E.W. Relaxivity studies on dinitroxide and polynitroxyl functionalized dendrimers: Effect of electron exchange and structure on paramagnetic relaxation enhancement. J. Phys. Chem. A 2003, 107, 8467–8475. [Google Scholar] [CrossRef]
- Zhang, S.; Lloveras, V.; Pulido, D.; Liko, F.; Pinto, L.F.; Albericio, F.; Royo, M.; Vidal-Gancedo, J. Radical Dendrimers based on biocompatible oligoethylene glycol dendrimers as contrast agents for MRI. Pharmaceutics 2020, 12, 772. [Google Scholar] [CrossRef] [PubMed]
- Francese, G.; Dunand, F.A.; Loosli, C.; Merbach, A.E.; Decurtins, S. Functionalization of PAMAM dendrimers with nitronyl nitroxide radicals as models for the outer-sphere relaxation in dentritic potential MRI contrast agents. Magn. Reson. Chem. 2003, 41, 81–83. [Google Scholar] [CrossRef]
- Rajca, A.; Wang, Y.; Boska, M.; Paletta, J.T.; Olankitwanit, A.; Swanson, M.A.; Mitchell, D.G.; Eaton, S.S.; Eaton, G.R.; Rajca, S. Organic radical contrast agents for magnetic resonance imaging. J. Am. Chem. Soc. 2012, 134, 15724–15727. [Google Scholar] [CrossRef] [Green Version]
- Pinto, L.F.; Lloveras, V.; Zhang, S.; Liko, F.; Veciana, J.; Muñoz-Gómez, J.L.; Vidal-Gancedo, J. Fully water-soluble polyphosphorhydrazone-based radical dendrimers functionalized with Tyr-PROXYL radicals as metal-free MRI T1 contrast agents. ACS Appl. Bio Mater. 2020, 3, 369–376. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Zhang, H.; Wu, Y. Dendrimer advances for the central nervous system delivery of therapeutics. ACS Chem. Neurosci. 2014, 5, 2–13. [Google Scholar] [CrossRef] [Green Version]
- Kitchens, K.M.; Foraker, A.B.; Kolhatkar, R.B.; Swaan, P.W.; Ghandehari, H. Endocytosis and interaction of poly (amidoamine) dendrimers with Caco-2 cells. Pharm. Res. 2007, 24, 2138–2145. [Google Scholar] [CrossRef]
- Sebestik, J.; Niederhafner, P.; Jezek, J. Peptide and glycopeptide dendrimers and analogous dendrimeric structures and their biomedical applications. Amino Acids 2011, 40, 301–370. [Google Scholar] [CrossRef]
- Luo, D.; Haverstick, K.; Belcheva, N.; Han, E.; Saltzman, W.M. Poly(ethylene glycol)-conjugated PAMAM dendrimer for biocompatible, high-efficiency DNA delivery. Macromolecules 2002, 35, 3456–3462. [Google Scholar] [CrossRef]
- Singh, P.; Gupta, U.; Asthana, A.; Jain, N.K. Folate and folate-PEG-PAMAM dendrimers: Synthesis, characterization, and targeted anticancer drug delivery potential in tumor bearing mice. Bioconjug. Chem. 2008, 19, 2239–2252. [Google Scholar] [CrossRef]
- Kojima, C.; Saito, K.; Kondo, E. Design of peptide–dendrimer conjugates with tumor homing and antitumor effects. Res. Chem. Intermed. 2018, 44, 4685–4695. [Google Scholar] [CrossRef]
- Kesharwani, P.; Gajbhiye, V.; Tekade, R.K.; Jain, N.K. Evaluation of dendrimer safety and efficacy through cell line studies. Curr. Drug Targets 2011, 12, 1478–1497. [Google Scholar] [CrossRef] [PubMed]
- Bhadra, D.; Yadav, A.K.; Bhadra, S.; Jain, N.K. Glycodendrimeric nanoparticulate carriers of primaquine phosphate for liver targeting. Int. J. Pharm. 2005, 295, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, P.; Gupta, U.; Jain, N.K. Glycoconjugated peptide dendrimers-based nanoparticulate system for the delivery of chloroquine phosphate. Biomaterials 2007, 28, 3349–3359. [Google Scholar] [CrossRef] [PubMed]
- Agashe, H.B.; Dutta, T.; Garg, M.; Jain, N.K. Investigations on the toxicological profile of functionalized fifth-generation poly(propylene imine) dendrimer. J. Pharm. Pharmacol. 2006, 58, 1491–1498. [Google Scholar] [CrossRef]
- Dobrovolskaia, M.A.; Patri, A.K.; Simak, J.; Hall, J.B.; Semberova, J.; De Paoli Lacerda, S.H.; McNeil, S.E. Nanoparticle size and surface charge determine effects of PAMAM dendrimers on human platelets in vitro. Mol. Pharm. 2012, 9, 382–393. [Google Scholar] [CrossRef] [Green Version]
- Enciso, A.; Neun, B.; Rodriguez, J.; Ranjan, A.; Dobrovolskaia, M.; Simanek, E. Nanoparticle effects on human platelets in vitro: A comparison between PAMAM and triazine dendrimers. Molecules 2016, 21, 428. [Google Scholar] [CrossRef]
- Spyropoulos-Antonakakis, N.; Sarantopoulou, E.; Trohopoulos, P.N.; Stefi, A.L.; Kollia, Z.; Gavriil, V.E.; Bourkoula, A.; Petrou, P.S.; Kakabakos, S.; Semashko, V.V.; et al. Selective aggregation of PAMAM dendrimer nanocarriers and PAMAM/ZnPc nanodrugs on human atheromatous carotid tissues: A photodynamic therapy for atherosclerosis. Nanoscale Res. Lett. 2015, 10. [Google Scholar] [CrossRef] [Green Version]
- Naha, P.C.; Davoren, M.; Lyng, F.M.; Byrne, H.J. Reactive oxygen species (ROS) induced cytokine production and cytotoxicity of PAMAM dendrimers in J774A.1 cells. Toxicol. Appl. Pharmacol. 2010, 246, 91–99. [Google Scholar] [CrossRef]
- Duncan, R.; Izzo, L. Dendrimer biocompatibility and toxicity. Adv. Drug Deliv. Rev. 2005, 57, 2215–2237. [Google Scholar] [CrossRef]
- Vidal, F.; Vásquez, P.; Cayumán, F.; Díaz, C.; Fuentealba, J.; Aguayo, L.; Yévenes, G.; Alderete, J.; Guzmán, L. Prevention of synaptic alterations and neurotoxic effects of PAMAM dendrimers by surface functionalization. Nanomaterials 2017, 8, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Zhu, H.; Wang, S.; Qian, X.; Fan, J.; Wang, Z.; Song, P.; Zhang, X.; Lu, W.; Ju, D. Interplay of oxidative stress and autophagy in PAMAM dendrimers-induced neuronal cell death. Theranostics 2015, 5, 1363–1377. [Google Scholar] [CrossRef] [Green Version]
- Hemmer, R.; Hall, A.; Spaulding, R.; Rossow, B.; Hester, M.; Caroway, M.; Haskamp, A.; Wall, S.; Bullen, H.A.; Morris, C.; et al. Analysis of biotinylated generation 4 poly(amidoamine) (PAMAM) dendrimer distribution in the rat brain and toxicity in a cellular model of the blood-brain barrier. Molecules 2013, 18, 11537–11552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, Y.; Kurokawa, Y.; Zeng, Q.; Win-Shwe, T.T.; Nansai, H.; Zhang, Z.; Sone, H. Effects of polyamidoamine dendrimers on a 3-D neurosphere system using human neural progenitor cells. Toxicol. Sci. 2016, 152, 128–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Win-Shwe, T.T.; Sone, H.; Kurokawa, Y.; Zeng, Y.; Zeng, Q.; Nitta, H.; Hirano, S. Effects of PAMAM dendrimers in the mouse brain after a single intranasal instillation. Toxicol. Lett. 2014, 228, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Yellepeddi, V.K.; Kumar, A.; Palakurthi, S. Surface modified poly(amido) amine dendrimers as diverse nanomolecules for biomedical applications. Expert Opin. Drug Deliv. 2009, 6, 835–850. [Google Scholar] [CrossRef]
- Kolhatkar, R.B.; Kitchens, K.M.; Swaan, P.W.; Ghandehari, H. Surface acetylation of polyamidoamine (PAMAM) dendrimers decreases cytotoxicity while maintaining membrane permeability. Bioconjug. +Chem. 2007, 18, 2054–2060. [Google Scholar] [CrossRef]
- Chauhan, A.; Kulhari, H. Pharmaceutical Applications of Dendrimers, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 251–269. [Google Scholar]
























© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chis, A.A.; Dobrea, C.; Morgovan, C.; Arseniu, A.M.; Rus, L.L.; Butuca, A.; Juncan, A.M.; Totan, M.; Vonica-Tincu, A.L.; Cormos, G.; et al. Applications and Limitations of Dendrimers in Biomedicine. Molecules 2020, 25, 3982. https://doi.org/10.3390/molecules25173982
Chis AA, Dobrea C, Morgovan C, Arseniu AM, Rus LL, Butuca A, Juncan AM, Totan M, Vonica-Tincu AL, Cormos G, et al. Applications and Limitations of Dendrimers in Biomedicine. Molecules. 2020; 25(17):3982. https://doi.org/10.3390/molecules25173982
Chicago/Turabian StyleChis, Adriana Aurelia, Carmen Dobrea, Claudiu Morgovan, Anca Maria Arseniu, Luca Liviu Rus, Anca Butuca, Anca Maria Juncan, Maria Totan, Andreea Loredana Vonica-Tincu, Gabriela Cormos, and et al. 2020. "Applications and Limitations of Dendrimers in Biomedicine" Molecules 25, no. 17: 3982. https://doi.org/10.3390/molecules25173982
APA StyleChis, A. A., Dobrea, C., Morgovan, C., Arseniu, A. M., Rus, L. L., Butuca, A., Juncan, A. M., Totan, M., Vonica-Tincu, A. L., Cormos, G., Muntean, A. C., Muresan, M. L., Gligor, F. G., & Frum, A. (2020). Applications and Limitations of Dendrimers in Biomedicine. Molecules, 25(17), 3982. https://doi.org/10.3390/molecules25173982