In Silico, In Vitro, and In Vivo Antitumor and Anti-Inflammatory Evaluation of a Standardized Alkaloid-Enriched Fraction Obtained from Boehmeria caudata Sw. Aerial Parts
Abstract
:1. Introduction
2. Results
2.1. Antiproliferative Assays
2.2. Cell Cycle Arrest and Clonogenic Cell Survival Assay
2.3. Molecular Docking
2.4. Phosphatidylserine Externalization and Caspases Activation
2.5. Acute Toxicity
2.6. Ehrlich Solid Tumor, Myeloperoxidase Activity, and Carrageenan-Induced Paw Edema Model
2.7. Croton Oil-Induced Ear Edema
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Plant Material
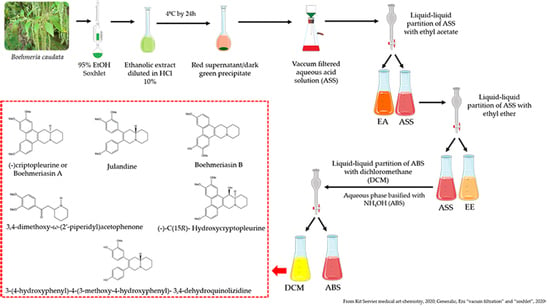
4.3. Production of the Alkaloid Enriched Fraction of B. caudate and Acid-Base Extraction
4.4. Cell Culture and Sample Preparation for In Vitro Experiments
4.5. Antiproliferative Activity
4.6. Colony Formation Assay
4.7. Cell Cycle Arrest
4.8. Phosphatidylserine Externalization
4.9. Detection of Activated Caspases
4.10. Molecular Docking Simulations
4.11. Animals and Drugs for In Vivo Experiments
4.12. Acute Toxicity Evaluation
4.13. Ehrlich Solid Tumor Model
4.14. Carrageenan-Induced Paw Edema
4.15. Croton Oil-Induced Ear Edema
4.16. Myeloperoxidase Activity Assay—Assessment of Neutrophil Activation/Migration
4.17. Statistical Analysis
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Aravindaram, K.; Yang, N.S. Anti-inflammatory plant natural products for cancer therapy. Planta Med. 2010, 76, 1103–1117. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, L.; Luo, Y.; Tian, M.; Zhang, S.-Y.; Lu, R.; Wang, J.-H.; Kasimu, R.; Li, X. Plant natural products: From traditional compounds to new emerging drugs in cancer therapy. Cell Prolif. 2014, 47, 506–515. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Lima, R.M.T.; Dos Reis, A.C.; De Menezes, A.-A.P.M.; Santos, J.V.D.O.; Filho, J.W.G.D.O.; Ferreira, J.R.D.O.; De Alencar, M.V.O.B.; Da Mata, A.M.O.F.; Khan, I.N.; Islam, A.; et al. Protective and therapeutic potential of ginger (Zingiber officinale) extract and [6]-gingerol in cancer: A comprehensive review. Phyther. Res. 2018, 32, 1885–1907. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Aragão, D.M.; De Assis Lima, I.V.; Da Silva, J.M.; Bellozi, P.M.Q.; De Carvalho da Costa, J.; Cardoso, G.M.M.; De Souza-Fagundes, E.M.; Scio, E. Anti-inflammatory, antinociceptive and cytotoxic effects of the methanol extract of Cecropia pachystachya Trécul. Phyther. Res. 2013, 27, 926–930. [Google Scholar] [CrossRef] [PubMed]
- De Paiva, P.P.; Nonato, F.R.; Ruiz, A.L.T.G.; De Oliveira Sousa, I.M.; Zafred, R.R.T.; De Oliveira, D.N.; Catharino, R.R.; Foglio, M.A.; De Carvalho, J.E. An ethanolic extract of Boehmeria caudata aerial parts displays anti-inflammatory and anti-tumor activities. Planta Medica Int. Open 2020, 7, e17–e25. [Google Scholar] [CrossRef] [Green Version]
- Prota, A.E.; Franck, D.; Bachmann, F.; Bargsten, K.; Buey, R.M.; Pohlmann, J.; Reinelt, S.; Lane, H.; Steinmetz, M.O. PDB Entry—4O2B. Available online: https://www.wwpdb.org/pdb?id=pdb_00004o2b (accessed on 28 August 2020).
- Mal’tseva, V.N.; Avkhacheva, N.V.; Santalov, B.F.; Safronova, V.G. Dynamic analysis of modification of peripheral neutrophils functional activity and its regulation during tumor growth in vivo. Tsitologiia 2006, 48, 1000–1009. [Google Scholar] [PubMed]
- National Cancer Institute NCI60 Methodology. Available online: https://dtp.cancer.gov/discovery_development/nci-60/methodology.htm (accessed on 12 August 2020).
- Paull, K.D.; Shoemaker, R.H.; Hodes, L.; Monks, A.; Scudiero, D.A.; Rubinstein, L.; Plowman, J.; Boyd, M.R. Display and analysis of patterns of differential activity of drugs against human tumor cell lines: Development of mean graph and COMPARE algorithm. J. Natl. Cancer Inst. 1989, 81, 1088–1092. [Google Scholar] [CrossRef] [PubMed]
- Zaharevitz, D.W.; Holbeck, S.L.; Bowerman, C.; Svetlik, P.A. COMPARE: A web accessible tool for investigating mechanisms of cell growth inhibition. J. Mol. Graph. Model. 2002, 20, 297–303. [Google Scholar] [CrossRef]
- Huang, R.; Wallqvist, A.; Covell, D.G. Assessment of in vitro and in vivo activities in the national cancer institute’s anticancer screen with respect to chemical structure, Target Specificity, and mechanism of action. J. Med. Chem. 2006, 49, 1964–1979. [Google Scholar] [CrossRef] [PubMed]
- Yue, Q.X.; Liu, X.; Guo, D.A. Microtubule-binding natural products for cancer therapy. Planta Med. 2010, 76, 1037–1043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molad, Y. Update on colchicine and its mechanism of action. Curr. Rheumatol. Rep. 2002, 4, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Muley, L.; Baum, B.; Smolinski, M.; Freindorf, M.; Heine, A.; Klebe, G.; Hangauer, D.G. Enhancement of hydrophobic interactions and hydrogen bond strength by cooperativity: Synthesis, modeling, and molecular dynamics simulations of a congeneric series of thrombin inhibitors. J. Med. Chem. 2010, 53, 2126–2135. [Google Scholar] [CrossRef]
- Bormio Nunes, J.H.; De Paiva, P.P.; Ruiz, A.L.T.G.; De Carvalho, J.E.; Corbi, P.P. New findings on the antiproliferative activity of the silver(I) complex with 5-fluorouracil against human multi-resistant NCI/ADR-RES ovarian tumor cells. Toxicol. Vitr. 2019, 60, 359–368. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef]
- De Carvalho Castro, K.N.; Lima, D.F.; Wolschick, D.; De Andrade, I.M.; Dos Santos, R.C.; De Seixas dos Santos, F.J.; Veras, L.M.C.; Costa-Júnior, L.M.; Veras, L.M.C.; Costa-Júnior, L.M. In vitro effects of Pilocarpus microphyllus extracts and pilocarpine hydrochloride on Rhipicephalus (Boophilus) microplus. Rev. Bras. Parasitol. Veterinária 2016, 25, 248–253. [Google Scholar] [CrossRef]
- De Araujo Furtado, M.; Rossetti, F.; Chanda, S.; Yourick, D. Exposure to nerve agents: From status epilepticus to neuroinflammation, brain damage, neurogenesis and epilepsy. Neurotoxicology 2012, 33, 1476–1490. [Google Scholar] [CrossRef]
- Yang, W.; Liao, G.; Hakim, S.G.; Ouyang, D.; Ringash, J.; Su, Y. Is pilocarpine effective in preventing radiation-induced xerostomia? A systematic review and meta-analysis. Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 503–511. [Google Scholar] [CrossRef] [Green Version]
- Saad, E.A.; Hassanien, M.M.; El-lban, F.W. Nickel(II) diacetyl monoxime-2-pyridyl hydrazone complex can inhibit Ehrlich solid tumor growth in mice: A potential new antitumor drug. Biochem. Biophys. Res. Commun. 2017, 484, 579–585. [Google Scholar] [CrossRef]
- Corso, C.R.; Acco, A. Glutathione system in animal model of solid tumors: From regulation to therapeutic target. Crit. Rev. Oncol. Hematol. 2018, 128, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Tamta, A.K.; Sarikhani, M.; Desingu, P.A.; Kizkekra, S.M.; Pandit, A.S.; Kumar, S.; Khan, D.; Raghavan, S.C.; Sundaresan, N.R. Subcutaneous Ehrlich Ascites Carcinoma mice model for studying cancer-induced cardiomyopathy. Sci. Rep. 2018, 8, 5599. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, R.A. The Biology of Cancer; Garland Publishing: New York, NY, USA, 2008. [Google Scholar]
- Vendramini-Costa, D.B.; De Carvalho, J.E. Molecular link mechanisms between inflammation and cancer. Curr. Pharm. Des. 2012, 18, 3831–3852. [Google Scholar] [CrossRef] [PubMed]
- Dumitru, C.A.; Lang, S.; Brandau, S. Modulation of neutrophil granulocytes in the tumor microenvironment: Mechanisms and consequences for tumor progression. Semin. Cancer Biol. 2013, 23, 141–148. [Google Scholar] [CrossRef]
- Liang, W.; Ferrara, N. The complex role of neutrophils in tumor angiogenesis and metastasis. Cancer Immunol. Res. 2016, 4, 83–91. [Google Scholar] [CrossRef] [Green Version]
- Moses, K.; Brandau, S. Human neutrophils: Their role in cancer and relation to myeloid-derived suppressor cells. Semin. Immunol. 2016, 28, 187–196. [Google Scholar] [CrossRef]
- Houghton, A.M. The paradox of tumor-associated neutrophils: Fueling tumor growth with cytotoxic substances. Cell Cycle 2010, 9, 1732–1737. [Google Scholar] [CrossRef] [Green Version]
- Winter, C.A.; Risley, E.A.; Nuss, G.W. Carrageenin-induced edema in hind paw of the rat as an assay for antiinflammatory drugs. Exp. Biol. Med. 1962, 111, 544–547. [Google Scholar] [CrossRef]
- Morris, C.J. Carrageenan-induced paw edema in the rat and mouse. In Methods in Molecular Biology (Clifton, N.J.); Humana Press: Totowa, NJ, USA, 2003; Volume 225, pp. 115–121. [Google Scholar]
- Vane, J.R.; Botting, R.M. New insights into the mode of action of anti-inflammatory drugs. Inflamm. Res. 1995, 44, 1–10. [Google Scholar] [CrossRef]
- Patil, K.R.; Mahajan, U.B.; Unger, B.S.; Goyal, S.N.; Belemkar, S.; Surana, S.J.; Ojha, S.; Patil, C.R. Animal models of inflammation for screening of anti-inflammatory drugs: Implications for the discovery and development of phytopharmaceuticals. Int. J. Mol. Sci. 2019, 20, 4367. [Google Scholar] [CrossRef] [Green Version]
- Tubaro, A.; Dri, P.; Melato, M.; Mulas, G.; Bianchi, P.; Negro, P.; Della Loggia, R. In the croton oil ear test the effects of non steroidal antiinflammatory drugs (NSAIDs) are dependent on the dose of the irritant. Agents Actions 1986, 19, 371–373. [Google Scholar] [CrossRef] [PubMed]
- Lapa, A.; Souccar, C.; Lima-Landman, M.; Castro, M.; Lima, T. Métodos de Avaliação da Atividade Farmacológica de Plantas Medicinais, 5th ed.; Setor de Produtos Naturais, Departamento de Farmacologia; UNIFESP/EPM: São Paulo, SP, Brazil, 2008. [Google Scholar]
- Fabri, R.L.; Garcia, R.A.; Florêncio, J.R.; De Castro Campos Pinto, N.; De Oliveira, L.G.; Aguiar, J.A.K.; Ribeiro, A.; Scio, E. Anti-inflammatory and antioxidative effects of the methanolic extract of the aerial parts of M itracarpus frigidus in established animal models. J. Pharm. Pharmacol. 2014, 66, 722–732. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.L.; Luft, C.; Lunardelli, A.; Amaral, R.H.; Melo, D.A.; Donadio, M.V.; Nunes, F.B.; Azambuja, M.S.; Santana, J.C.; Moraes, C.; et al. Antioxidant, analgesic and anti-inflammatory effects of lavender essential oil. An. Acad. Bras. Cienc. 2015, 87, 1397–1408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlson, R.P.; Lynn, O.D.; Chang, J.; Lewis, A.J. Modulation of mouse ear edema by cyclooxygenase and lipoxygenase inhibitors and other pharmacologic agents. Agents Actions 1985, 17, 197–204. [Google Scholar] [CrossRef]
- Yang, C.W.; Chen, W.L.; Wu, P.L.; Tseng, H.Y.; Lee, S.J. Anti-inflammatory mechanisms of phenanthroindolizidine alkaloids. Mol. Pharmacol. 2006, 69, 749–758. [Google Scholar] [CrossRef] [Green Version]
- Chemler, S.R. Phenanthroindolizidines and phenanthroquinolizidines: Promising alkaloids for anti-cancer therapy. Curr. Bioact. Compd. 2009, 5, 2–19. [Google Scholar] [CrossRef]
- Hashmi, M.A.; Khan, A.; Farooq, U.; Khan, S. Alkaloids as Cyclooxygenase Inhibitors in Anticancer Drug Discovery. Curr. Protein Pept. Sci. 2018, 19, 292–301. [Google Scholar] [CrossRef]
- Queiroz, R.F.; Jordão, A.K.; Cunha, A.C.; Ferreira, V.F.; Brigagão, M.R.P.L.; Malvezzi, A.; Amaral, A.T.D.; Augusto, O. Nitroxides attenuate carrageenan-induced inflammation in rat paws by reducing neutrophil infiltration and the resulting myeloperoxidase-mediated damage. Free Radic. Biol. Med. 2012, 53, 1942–1953. [Google Scholar] [CrossRef]
- Bormio Nunes, J.H.; Bergamini, F.R.G.; Lustri, W.R.; Paiva, P.P.; Ruiz, A.L.T.G.; Carvalho, J.E.; Corbi, P.P. Synthesis, characterization and in vitro biological assays of a silver(I) complex with 5-fluorouracil: A strategy to overcome multidrug resistant tumor cells. J. Fluor. Chem. 2017, 195, 93–101. [Google Scholar] [CrossRef]
- Bormio Nunes, J.H.; Simoni, D.A.; Braga, L.E.O.; Ruiz, A.L.T.G.; Ernesto de Carvalho, J.; Corbi, P.P. Synthesis, characterization, crystal structure and in vitro antiproliferative assays of the 2-thiouracilato(triphenylphosphine)gold(I) complex. J. Mol. Struct. 2019, 1178, 169–178. [Google Scholar] [CrossRef]
- National Cancer Institute Cell Lines in the In Vitro Screen. Available online: https://dtp.cancer.gov/discovery_development/nci-60/cell_list.htm (accessed on 5 August 2019).
- Franco, Y.E.M.; Okubo, M.Y.; Torre, A.D.; Paiva, P.P.; Rosa, M.N.; Silva, V.A.O.; Reis, R.M.; Ruiz, A.L.T.G.; Imamura, P.M.; De Carvalho, J.E.; et al. Coronarin D induces apoptotic cell death and cell cycle arrest in human glioblastoma cell line. Molecules 2019, 24, 4498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neto, X.A.D.O.; Alves, A.C.S.; Junior, R.A.D.; Rodrigues, R.P.; Lancellotti, M.; Almeida, W.P.; Kawano, D.F. Molecular docking reveals the binding modes of anticancer alkylphospholipids and lysophosphatidylcholine within the catalytic domain of cytidine triphosphate: Phosphocholine cytidyltransferase. Eur. J. Lipid Sci. Technol. 2020, 122, 1900422. [Google Scholar] [CrossRef]
- De Oliveira, J.F.; Da Silva, A.L.; Vendramini-Costa, D.B.; Da Cruz Amorim, C.A.; Campos, J.F.; Ribeiro, A.G.; De Moura, R.O.; Neves, J.L.; Ruiz, A.L.T.G.; De Carvalho, J.E.; et al. Synthesis of thiophene-thiosemicarbazone derivatives and evaluation of their in vitro and in vivo antitumor activities. Eur. J. Med. Chem. 2015, 104, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Yen, M.H.; Lo, T.S.; Lin, C.F. The antiinflammatory and liver protective effects of Boehmeria nivea and B. nivea subsp. nippononivea in rats. Phytomedicine 1997, 4, 301–308. [Google Scholar] [CrossRef]
- Bradley, P.P.; Priebat, D.A.; Christensen, R.D.; Rothstein, G. Measurement of Cutaneous Inflammation: Estimation of Neutrophil Content with an Enzyme Marker. J. Investig. Dermatol. 1982, 78, 206–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Sample Availability: Samples of the compounds are not available from the authors. |






| Compounds | Cell Lines * | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2 | M | A | 7 | 4 | P | O | H | K | Cat | |
| BcAEF | 0.59 | 0.61 | >2.5 | >2.5 | 0.17 | 0.32 | 0.79 | 0.24 | 0.11 | 0.19 |
| Doxorubicin | >25 | 2.83 | >25 | 1.88 | >25 | 0.83 | 1.23 | >25 | 0.93 | 0.78 |
| Route of Administration | Group a | ||
|---|---|---|---|
| Vehicle b | Dexamethasone c | BcAEF d | |
| Topic application | 20 µL | 5 mg/mL | 3,10, 30 mg/mL |
| Oral administration | 10 mL/kg | 25 mg/kg | 3,10, 30 mg/kg |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paiva, P.P.d.; Nunes, J.H.B.; Nonato, F.R.; Ruiz, A.L.T.G.; Zafred, R.R.T.; Sousa, I.M.O.; Okubo, M.Y.; Kawano, D.F.; Monteiro, P.A.; Foglio, M.A.; et al. In Silico, In Vitro, and In Vivo Antitumor and Anti-Inflammatory Evaluation of a Standardized Alkaloid-Enriched Fraction Obtained from Boehmeria caudata Sw. Aerial Parts. Molecules 2020, 25, 4018. https://doi.org/10.3390/molecules25174018
Paiva PPd, Nunes JHB, Nonato FR, Ruiz ALTG, Zafred RRT, Sousa IMO, Okubo MY, Kawano DF, Monteiro PA, Foglio MA, et al. In Silico, In Vitro, and In Vivo Antitumor and Anti-Inflammatory Evaluation of a Standardized Alkaloid-Enriched Fraction Obtained from Boehmeria caudata Sw. Aerial Parts. Molecules. 2020; 25(17):4018. https://doi.org/10.3390/molecules25174018
Chicago/Turabian StylePaiva, Paula P. de, Julia H. B. Nunes, Fabiana R. Nonato, Ana L. T. G. Ruiz, Rafael R. T. Zafred, Ilza M. O. Sousa, Márcia Y. Okubo, Daniel F. Kawano, Paula A. Monteiro, Mary A. Foglio, and et al. 2020. "In Silico, In Vitro, and In Vivo Antitumor and Anti-Inflammatory Evaluation of a Standardized Alkaloid-Enriched Fraction Obtained from Boehmeria caudata Sw. Aerial Parts" Molecules 25, no. 17: 4018. https://doi.org/10.3390/molecules25174018
APA StylePaiva, P. P. d., Nunes, J. H. B., Nonato, F. R., Ruiz, A. L. T. G., Zafred, R. R. T., Sousa, I. M. O., Okubo, M. Y., Kawano, D. F., Monteiro, P. A., Foglio, M. A., & Carvalho, J. E. (2020). In Silico, In Vitro, and In Vivo Antitumor and Anti-Inflammatory Evaluation of a Standardized Alkaloid-Enriched Fraction Obtained from Boehmeria caudata Sw. Aerial Parts. Molecules, 25(17), 4018. https://doi.org/10.3390/molecules25174018