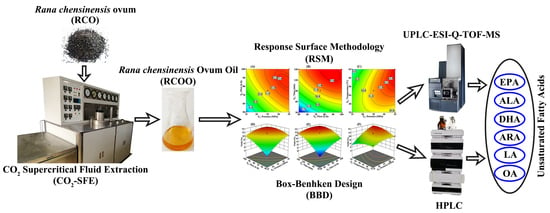
Rana chensinensis Ovum Oil Based on CO2 Supercritical Fluid Extraction: Response Surface Methodology Optimization and Unsaturated Fatty Acid Ingredient Analysis
Abstract
:1. Introduction
2. Results and Discussions
2.1. Adaptability Evaluation of BBD Model
2.2. The Influence of Various Factors in the BBD Model
2.3. Improvement of Yield Based on the BBD Model
2.4. Identification of Principal UFAs of RCOO
2.5. Yield Analysis of Principal UFAs
3. Materials and Methods
3.1. Reagents and Samples
3.2. Sample Preparation
3.3. CO2-SFE Procedure
3.4. Experimental Design of RSM Based on BBD
3.5. UPLC-ESI-Q-TOF-MS Component Analysis
3.6. HPLC Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gan, Y.; Xiao, Y.; Wang, S.; Guo, H.; Liu, M.; Wang, Z.; Wang, Y. Protein-based fingerprint analysis for the identification of Ranae Oviductus using RP-HPLC. Molecules 2019, 24, 1687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Xu, Y.; Wang, Y.; Lv, Z.; Cui, Q.; Jin, X.; Wang, Y. HPLC fingerprint combined with quantitation of main effective components and chemometrics as an efficient method for quality evaluation of Oviductus Ranae. Nat. Prod. Commun. 2017, 12, 1495–1499. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.; Ni, S.; Wang, S.; Gan, Y.; Zhou, Y.; Guo, H.; Liu, M.; Wang, Z.; Wang, Y. Environmental influences on quality features of Oviductus Ranae in the Changbai Mountains. RSC Adv. 2019, 9, 36050–36057. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Wang, F.; Guo, H.; Wang, S.; Ni, S.; Zhou, Y.; Wang, Z.; Bao, H.; Wang, Y. Antitussive and anti-inflammatory dual-active agents developed from natural product lead compound 1-methylhydantoin. Molecules 2019, 24, 2355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Xu, Y.; Wang, Y.; Yang, H.; Lv, Z.; Jin, X.; Wang, Y. Simultaneous determination of six active components in Oviductus Ranae via quantitative analysis of multicomponents by single marker. J. Anal. Methods Chem. 2017, 2017, 9194847. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Wang, S.; Luo, Y.; Wang, Y.; Qu, X. Evaluation of the merits of the new method of Oviductus Ranae by HPLC-DAD. J. Liq. Chromatogr. Relat. Technol. 2015, 38, 1218–1222. [Google Scholar] [CrossRef]
- Guo, H.; Gan, Y.; Liu, M.; Wang, S.; Ni, S.; Zhou, Y.; Xiao, Y.; Wang, Z.; Wang, Y. Quality evaluation of Oviductus Ranae based on PUFAs using HPLC fingerprint techniques combined with chemometric methods. Foods 2019, 8, 322. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhao, H.; Zhang, Y. Research progress in active ingredient extraction methods and pharmacological effects of skin and eggs derived from Rana chensinensis. J. Jilin Inst. Chem. Technol. 2019, 36, 15–20. [Google Scholar]
- Yin, Y.; Chen, N.; Chang, L.; Gen, X.; Wei, G. Antioxidant and moisturizing ability of fatty acids from ovum oil of Rana dybowskii. J. Econ. Animal 2018, 22, 30–36. [Google Scholar]
- Kris-Etherton, P.M.; Grieger, J.A.; Etherton, T.D. Dietary reference intakes for DHA and EPA. Prostaglandins Leukot. Essent Fat. Acids 2009, 81, 99–104. [Google Scholar] [CrossRef]
- Hamilton, H.A.; Newton, R.; Auchterlonie, N.A.; Müller, D.B. Systems approach to quantify the global omega-3 fatty acid cycle. Nat. Food 2020, 1, 59–62. [Google Scholar] [CrossRef] [Green Version]
- Abedi, E.; Sahari, M.A. Long-chain polyunsaturated fatty acid sources and evaluation of their nutritional and functional properties. Food Sci. Nutr. 2014, 2, 443–463. [Google Scholar] [CrossRef] [PubMed]
- Schacky, C.; Harris, W.S. Cardiovascular benefits of omega-3 fatty acids. Cardiovasc. Res. 2007, 73, 310–315. [Google Scholar]
- Hou, D.; Diao, Q.; Sun, B.; Hui, R. Analysis of fatty acids in Rana chensinensis eggs by two different extraction methods combined with GC-MS. Food Sci. 2013, 33, 195–197. [Google Scholar]
- Bao, X.; Huang, J.; Wang, Y.; Hou, B.; Sun, Z.; Yan, L. Analysis of influencing factors on the extraction rate of the oil of Rana chensinensis ovum. FuJian Agric. 2015, 15, 154–155. [Google Scholar]
- Manirakiza, P.; Covaci, A.; Schepens, P. Comparative study on total lipid determination using Soxhlet, Roese-Gottlieb, Bligh & Dyer, and modified Bligh & Dyer extraction methods. J. Food Compos. Anal. 2001, 14, 93–100. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Ou, H.; Gregersen, H. Ultrasound-assisted supercritical CO2 extraction of cucurbitacin E from Iberis amara seeds. Ind. Crop. Prod. 2020, 145, 112093. [Google Scholar] [CrossRef]
- Nagavekar, N.; Singhal, R.S. Simultaneous extraction of flaxseed spice blend using supercritical carbon dioxide: Process optimization, bioactivity profile, and application as a functional seasoning. Sep. Purif. Technol. 2020, 248, 117030. [Google Scholar] [CrossRef]
- Cunico, L.P.; Turner, C. Chapter 7—Supercritical fluids and gas-expanded liquids. In The Application of Green Solvents in Separation Processes; Pena-Pereira, F., Tobiszewski, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 155–214. [Google Scholar]
- Zhao, S.; Zhang, D. A parametric study of supercritical carbon dioxide extraction of oil from Moringa oleifera seeds using a response surface methodology. Sep. Purif. Technol. 2013, 113, 9–17. [Google Scholar] [CrossRef]
- Baş, D.; Boyacı, İ.H. Modeling and optimization I: Usability of response surface methodology. J. Food Eng. 2007, 78, 836–845. [Google Scholar] [CrossRef]
- Sharif, K.M.; Rahman, M.M.; Azmir, J.; Mohamed, A.; Jahurul, M.H.A.; Sahena, F.; Zaidul, I.S.M. Experimental design of supercritical fluid extraction—A review. J. Food Eng. 2014, 124, 105–116. [Google Scholar] [CrossRef]
- Liu, Z.; Mei, L.; Wang, Q.; Shao, Y.; Tao, Y. Optimization of subcritical fluid extraction of seed oil from Nitraria tangutorum using response surface methodology. LWT Food Sci. Technol. 2014, 56, 168–174. [Google Scholar] [CrossRef]
- Pujari, V.; Chandra, T.S. Statistical optimization of medium components for enhanced riboflavin production by a UV-mutant of Eremothecium ashbyii. Process. Biochem. 2000, 36, 31–37. [Google Scholar] [CrossRef]
- Şanal, İ.S.; Bayraktar, E.; Mehmetoğlu, Ü.; Çalımlı, A. Determination of optimum conditions for SC-(CO2 + ethanol) extraction of β-carotene from apricot pomace using response surface methodology. J. Supercrit. Fluids 2005, 34, 331–338. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Q.; Qiao, G.; Qiu, Z.; Wen, Z.; Wen, X. Optimizing the supercritical carbon dioxide extraction of sweet cherry (Prunus avium L.) leaves and UPLC-MS/MS analysis. Anal. Methods 2020, 12, 3004–3013. [Google Scholar] [CrossRef]
- Liu, S.; Yang, F.; Zhang, C.; Ji, H.; Hong, P.; Deng, C. Optimization of process parameters for supercritical carbon dioxide extraction of Passiflora seed oil by response surface methodology. J. Supercrit. Fluids 2009, 48, 9–14. [Google Scholar] [CrossRef]
- Vohra, A.; Satyanarayana, T. Statistical optimization of the medium components by response surface methodology to enhance phytase production by Pichia anomala. Process. Biochem. 2002, 37, 999–1004. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Wei, S.; Yan, Z. Application of response surface methodology to optimise supercritical carbon dioxide extraction of essential oil from Cyperus rotundus Linn. Food Chem. 2012, 132, 582–587. [Google Scholar] [CrossRef]
- Muralidhar, R.V.; Chirumamila, R.R.; Marchant, R.; Nigam, P. A response surface approach for the comparison of lipase production by Candida cylindracea using two different carbon sources. Biochem. Eng. J. 2001, 9, 17–23. [Google Scholar] [CrossRef]
- Rozzi, N.L.; Singh, R.K.; Vierling, R.A.; Watkins, B.A. Supercritical fluid extraction of lycopene from tomato processing byproducts. J. Agric. Food Chem. 2002, 50, 2638–2643. [Google Scholar] [CrossRef] [PubMed]
- Thana, P.; Machmudah, S.; Goto, M.; Sasaki, M.; Pavasant, P.; Shotipruk, A. Response surface methodology to supercritical carbon dioxide extraction of astaxanthin from Haematococcus pluvialis. Bioresour. Technol. 2008, 99, 3110–3115. [Google Scholar] [CrossRef] [PubMed]
- Durante, M.; Ferramosca, A.; Treppiccione, L.; Di Giacomo, M.; Zara, V.; Montefusco, A.; Piro, G.; Mita, G.; Bergamo, P.; Lenucci, M.S. Application of response surface methodology (RSM) for the optimization of supercritical CO2 extraction of oil from patè olive cake: Yield, content of bioactive molecules and biological effects in vivo. Food Chem. 2020, 332, 127405. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.; Frederich, M.; Megido, R.C.; Alabi, T.; Malik, P.; Uyttenbroeck, R.; Francis, F.; Blecker, C.; Haubruge, E.; Lognay, G.; et al. Insect fatty acids: A comparison of lipids from three Orthopterans and Tenebrio molitor L. larvae. J. Asia Pac. Entomol. 2017, 20, 337–340. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.-S. Omega-3 and omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance—A review. Life Sci. 2018, 203, 255–267. [Google Scholar] [CrossRef]
- Schmitz, G.; Ecker, J. The opposing effects of n−3 and n−6 fatty acids. Prog. Lipid Res. 2008, 47, 147–155. [Google Scholar] [CrossRef]
- Jimenez-Lopez, C.; Carpena, M.; Lourenço-Lopes, C.; Gallardo-Gomez, M.; Lorenzo, J.M.; Barba, F.J.; Prieto, M.A.; Simal-Gandara, J. Bioactive compounds and quality of extra virgin olive oil. Foods 2020, 9, 1014. [Google Scholar] [CrossRef]
- Różańska, A.; Russo, M.; Cacciola, F.; Salafia, F.; Polkowska, Ż.; Dugo, P.; Mondello, L. Concentration of potentially bioactive compounds in italian extra virgin olive oils from various sources by using LC-MS and multivariate data analysis. Foods 2020, 9, 1120. [Google Scholar] [CrossRef]
- Wrona, O.; Rafińska, K.; Możeński, C.; Buszewski, B. Optimization and upscaling of the supercritical carbon dioxide extraction of Solidago gigantea Ait. of an industrial relevance. Ind. Crop. Prod. 2019, 142, 111787. [Google Scholar] [CrossRef]
- Marić, B.; Pavlić, B.; Čolović, D.; Abramović, B.; Zeković, Z.; Bodroža-Solarov, M.; Ilić, N.; Teslić, N. Recovery of high-content ω–3 fatty acid oil from raspberry (Rubus idaeus L.) seeds: Chemical composition and functional quality. LWT Food Sci. Technol. 2020, 130, 109627. [Google Scholar]
- Lenucci, M.S.; Caccioppola, A.; Durante, M.; Serrone, L.; Leonardo, R.; Piro, G.; Dalessandro, G. Optimisation of biological and physical parameters for lycopene supercritical CO2 extraction from ordinary and high-pigment tomato cultivars. J. Sci. Food Agric. 2010, 90, 1709–1718. [Google Scholar] [CrossRef] [PubMed]
- Maran, J.P.; Manikandan, S.; Priya, B.; Gurumoorthi, P. Box-Behnken design based multi-response analysis and optimization of supercritical carbon dioxide extraction of bioactive flavonoid compounds from tea (Camellia sinensis L.) leaves. J. Food Sci. Technol. 2015, 52, 92–104. [Google Scholar] [CrossRef]
Sample Availability: Not available. |





| No. | X1: Pressure (MPa) | X2: Flow (L/h) | X3: Temperature (°C) | X4: Time (min) | Actual Yield (%) | Predicted Yield (%) | Residual (%) |
|---|---|---|---|---|---|---|---|
| SFE1 | 35 | 75 | 47 | 30 | 8.69 | 8.05 | 0.64 |
| SFE2 | 25 | 75 | 47 | 90 | 11.48 | 11.31 | 0.17 |
| SFE3 | 35 | 100 | 47 | 90 | 11.92 | 12.19 | −0.27 |
| SFE4 | 25 | 100 | 32 | 90 | 11.99 | 11.36 | 0.63 |
| SFE5 | 15 | 100 | 47 | 90 | 9.08 | 10.09 | −1.01 |
| SFE6 | 25 | 100 | 62 | 90 | 11.69 | 10.61 | 1.08 |
| SFE7 | 25 | 50 | 47 | 150 | 11.51 | 11.12 | 0.39 |
| SFE8 | 35 | 75 | 32 | 90 | 10.16 | 9.65 | 0.51 |
| SFE9 | 25 | 75 | 32 | 30 | 3.81 | 5.20 | −1.39 |
| SFE10 | 35 | 75 | 47 | 150 | 13.06 | 13.42 | −0.36 |
| SFE11 | 15 | 75 | 47 | 30 | 3.03 | 1.92 | 1.11 |
| SFE12 | 25 | 50 | 62 | 90 | 7.55 | 7.42 | 0.13 |
| SFE13 | 25 | 75 | 47 | 90 | 11.55 | 11.31 | 0.24 |
| SFE14 | 25 | 75 | 62 | 150 | 12.56 | 12.20 | 0.36 |
| SFE15 | 25 | 50 | 47 | 30 | 2.36 | 1.77 | 0.59 |
| SFE16 | 25 | 100 | 47 | 30 | 7.96 | 8.07 | −0.11 |
| SFE17 | 15 | 75 | 47 | 150 | 10.65 | 10.53 | 0.12 |
| SFE18 | 25 | 75 | 47 | 90 | 10.89 | 11.31 | −0.42 |
| SFE19 | 15 | 75 | 32 | 90 | 9.24 | 8.48 | 0.76 |
| SFE20 | 25 | 75 | 32 | 150 | 11.25 | 11.44 | −0.19 |
| SFE21 | 25 | 100 | 47 | 150 | 12.39 | 12.71 | −0.32 |
| SFE22 | 25 | 75 | 62 | 30 | 3.61 | 4.46 | −0.85 |
| SFE23 | 35 | 50 | 47 | 90 | 10.62 | 10.65 | −0.03 |
| SFE24 | 15 | 50 | 47 | 90 | 2.98 | 3.74 | −0.76 |
| SFE25 | 35 | 75 | 62 | 90 | 12.51 | 13.00 | −0.49 |
| SFE26 | 15 | 75 | 62 | 90 | 4.93 | 5.16 | −0.23 |
| SFE27 | 25 | 50 | 32 | 90 | 6.32 | 6.65 | −0.33 |
| Source | Coefficient Estimate | Sum of Squares | df | Mean Square | F-value | p-value | Significance |
|---|---|---|---|---|---|---|---|
| Model | N/A | 301.02 | 14 | 21.50 | 25.91 | <0.0001 | ** |
| Intercept | 11.31 | N/A | N/A | N/A | N/A | N/A | N/A |
| X1: Pressure | 2.25 | 60.98 | 1 | 60.98 | 73.49 | <0.0001 | ** |
| X2: Flow | 1.97 | 46.77 | 1 | 46.77 | 56.36 | <0.0001 | ** |
| X3: Temperature | 0.007 | 0.0005 | 1 | 0.0005 | 0.0006 | 0.9802 | not significant |
| X4: Time | 3.50 | 146.72 | 1 | 146.72 | 176.82 | <0.0001 | ** |
| X1 X2 | −1.20 | 5.76 | 1 | 5.76 | 6.94 | 0.0218 | * |
| X1X3 | 1.67 | 11.09 | 1 | 11.09 | 13.36 | 0.0033 | ** |
| X1X4 | −0.81 | 2.64 | 1 | 2.64 | 3.18 | 0.0997 | not significant |
| X2X3 | −0.38 | 0.59 | 1 | 0.59 | 0.71 | 0.4174 | not significant |
| X2X4 | −1.18 | 5.57 | 1 | 5.57 | 6.71 | 0.0236 | * |
| X3X4 | 0.38 | 0.57 | 1 | 0.57 | 0.69 | 0.4234 | not significant |
| X12 | −1.04 | 5.76 | 1 | 5.76 | 6.94 | 0.0218 | * |
| X22 | −1.10 | 6.47 | 1 | 6.47 | 7.80 | 0.0163 | * |
| X32 | −1.20 | 7.62 | 1 | 7.62 | 9.19 | 0.0105 | * |
| X42 | −1.79 | 17.05 | 1 | 17.05 | 20.55 | 0.0007 | ** |
| Residual | N/A | 9.96 | 12 | 0.83 | N/A | N/A | N/A |
| Lack of Fit | N/A | 9.69 | 10 | 0.97 | 7.38 | 0.1252 | not significant |
| Pure Error | N/A | 0.26 | 2 | 0.13 | N/A | N/A | N/A |
| Cor Total | N/A | 310.98 | 26 | N/A | N/A | N/A | N/A |
| R2 = 0.9680 | Adjusted R2 = 0.9306 | Predicted R2 = 0.8185 | Adeq Precision = 17.1612 | ||||
| Peak | RT in MS a (min) | [M − H]− | Mass Error (mDa) | Molecular Formula | Proposed Compound | RT in HPLC b (min) |
|---|---|---|---|---|---|---|
| 1 | 1.287 | 301.1901 | 2.0 | C20H30O2 | EPA | 10.29 |
| 2 | 1.400 | 277.1917 | 2.3 | C18H30O2 | ALA | 11.26 |
| 3 | 1.467 | 327.2035 | 1.9 | C22H32O2 | DHA | 12.19 |
| 4 | 1.614 | 303.2077 | 2.5 | C20H32O2 | ARA | 13.91 |
| 5 | 1.806 | 279.2090 | 2.8 | C18H32O2 | LA | 15.66 |
| 6 | 2.427 | 281.2235 | 2.1 | C18H34O2 | OA | 22.53 |
| Coding Level | Pressure (X1, MPa) | Flow (X2, L/h) | Temperature (X3, °C) | Time (X4, min) |
|---|---|---|---|---|
| −1 | 15 | 50 | 32 | 30 |
| 0 | 25 | 75 | 47 | 90 |
| +1 | 35 | 100 | 62 | 150 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gan, Y.; Xu, D.; Zhang, J.; Wang, Z.; Wang, S.; Guo, H.; Zhang, K.; Li, Y.; Wang, Y. Rana chensinensis Ovum Oil Based on CO2 Supercritical Fluid Extraction: Response Surface Methodology Optimization and Unsaturated Fatty Acid Ingredient Analysis. Molecules 2020, 25, 4170. https://doi.org/10.3390/molecules25184170
Gan Y, Xu D, Zhang J, Wang Z, Wang S, Guo H, Zhang K, Li Y, Wang Y. Rana chensinensis Ovum Oil Based on CO2 Supercritical Fluid Extraction: Response Surface Methodology Optimization and Unsaturated Fatty Acid Ingredient Analysis. Molecules. 2020; 25(18):4170. https://doi.org/10.3390/molecules25184170
Chicago/Turabian StyleGan, Yuanshuai, Dongliang Xu, Jianqiu Zhang, Zhongyao Wang, Shihan Wang, Hongye Guo, Kexin Zhang, Yajing Li, and Yongsheng Wang. 2020. "Rana chensinensis Ovum Oil Based on CO2 Supercritical Fluid Extraction: Response Surface Methodology Optimization and Unsaturated Fatty Acid Ingredient Analysis" Molecules 25, no. 18: 4170. https://doi.org/10.3390/molecules25184170
APA StyleGan, Y., Xu, D., Zhang, J., Wang, Z., Wang, S., Guo, H., Zhang, K., Li, Y., & Wang, Y. (2020). Rana chensinensis Ovum Oil Based on CO2 Supercritical Fluid Extraction: Response Surface Methodology Optimization and Unsaturated Fatty Acid Ingredient Analysis. Molecules, 25(18), 4170. https://doi.org/10.3390/molecules25184170