Cyanobacterial Polyhydroxyalkanoates: A Sustainable Alternative in Circular Economy
Abstract
:1. Introduction
2. Bioplastics
2.1. Polyhydroxyalkanoates
2.1.1. PHA Structure
2.1.2. PHA Biosynthetic Pathways
2.1.3. Physical and Mechanical Properties
2.1.4. Applications
3. Cyanobacteria
3.1. PHA Production in Cyanobacteria
3.2. Waste Utilization and Bioremediation
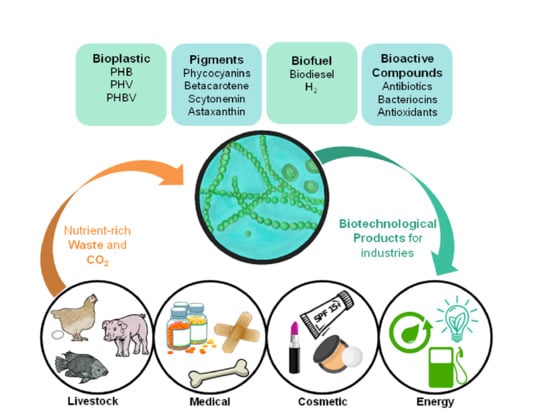
4. Cyanobacteria Potential Application in Circular Economy
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Plastics—The Facts 2019. Available online: https://issuu.com/plasticseuropeebook/docs/final_web_version_plastics_the_facts2019_14102019 (accessed on 24 June 2020).
- US EPA Plastics: Material-Specific Data. Available online: https://www.epa.gov/facts-and-figures-about-materials-waste-and-recycling/plastics-material-specific-data (accessed on 24 June 2020).
- Koller, M.; Hesse, P.; Kutschera, C.; Bona, R.; Nascimento, J.; Ortega, S.; Agnelli, J.A.; Braunegg, G. Sustainable Embedding of the Bioplastic Poly-(3-Hydroxybutyrate) into the Sugarcane Industry: Principles of a Future-Oriented Technology in Brazil. In Polymers-Opportunities and Risks II; Eyerer, P., Weller, M., Hübner, C., Eds.; Springer: Berlin, Germany, 2009; Volume 12, pp. 81–96. ISBN 9783642027963. [Google Scholar]
- Carpenter, E.J.; Smith, K.L. Plastics on the Sargasso Sea Surface. Science 1972, 175, 1240–1241. [Google Scholar] [CrossRef] [PubMed]
- Dhaman, Y.; Ugwu, C.U. Poly[(R)-3-hydroxybutyrate]: The Green Biodegradable Bioplastics of the Future! Ferment. Technol 2012, 01. [Google Scholar] [CrossRef]
- Philip, S.; Keshavarz, T.; Roy, I. Polyhydroxyalkanoates: Biodegradable polymers with a range of applications. J. Chem. Technol. Biotechnol. 2007, 82, 233–247. [Google Scholar] [CrossRef]
- Wu, G.F.; Wu, Q.Y.; Shen, Z.Y. Accumulation of poly-β-hydroxybutyrate in cyanobacterium Synechocystis sp. PCC6803. Bioresour. Technol. 2001, 76, 85–90. [Google Scholar] [CrossRef]
- Akaraonye, E.; Keshavarz, T.; Roy, I. Production of polyhydroxyalkanoates: The future green materials of choice. J. Chem. Technol. Biotechnol. 2010, 85, 732–743. [Google Scholar] [CrossRef]
- Karan, H.; Funk, C.; Grabert, M.; Oey, M.; Hankamer, B. Green Bioplastics as Part of a Circular Bioeconomy. Trends Plant. Sci. 2019, 24, 237–249. [Google Scholar] [CrossRef]
- Choi, J.; Lee, S.Y. Factors affecting the economics of polyhydroxyalkanoate production by bacterial fermentation. Appl. Microbiol. Biotechnol. 1999, 51, 13–21. [Google Scholar] [CrossRef]
- Lee, G.; Na, J. Future of microbial polyesters. Microb. Cell Fact. 2013, 12, 54. [Google Scholar] [CrossRef] [Green Version]
- Balaji, S.; Gopi, K.; Muthuvelan, B. A review on production of poly β hydroxybutyrates from cyanobacteria for the production of bio plastics. Algal Res. 2013, 2, 278–285. [Google Scholar] [CrossRef]
- Singh, A.K.; Mallick, N. Advances in cyanobacterial polyhydroxyalkanoates production. FEMS Microbiol. Lett. 2017, 364. [Google Scholar] [CrossRef]
- Drosg, B.; Fritz, I.; Gattermyer, F.; Silvestrini, L. Photo-autotrophic Production of Poly(hydroxyalkanoates) in Cyanobacteria. Chem. Biochem. Eng. Q. 2015, 29, 145–156. [Google Scholar] [CrossRef]
- Arias, D.M.; García, J.; Uggetti, E. Production of polymers by cyanobacteria grown in wastewater: Current status, challenges and future perspectives. New Biotechnol. 2020, 55, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Pittman, J.K.; Dean, A.P.; Osundeko, O. The potential of sustainable algal biofuel production using wastewater resources. Bioresour. Technol. 2011, 102, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Rawat, I.; Ranjith Kumar, R.; Mutanda, T.; Bux, F. Dual role of microalgae: Phycoremediation of domestic wastewater and biomass production for sustainable biofuels production. Appl. Energy 2011, 88, 3411–3424. [Google Scholar] [CrossRef]
- Stiles, W.A.V.; Styles, D.; Chapman, S.P.; Esteves, S.; Bywater, A.; Melville, L.; Silkina, A.; Lupatsch, I.; Fuentes Grünewald, C.; Lovitt, R.; et al. Using microalgae in the circular economy to valorise anaerobic digestate: Challenges and opportunities. Bioresour. Technol. 2018, 267, 732–742. [Google Scholar] [CrossRef] [Green Version]
- Lai, Y.-C.; Chang, C.-H.; Chen, C.-Y.; Chang, J.-S.; Ng, I.-S. Towards protein production and application by using Chlorella species as circular economy. Bioresour. Technol. 2019, 289, 121625. [Google Scholar] [CrossRef]
- Blank, L.M.; Narancic, T.; Mampel, J.; Tiso, T.; O’Connor, K. Biotechnological upcycling of plastic waste and other non-conventional feedstocks in a circular economy. Curr. Opin. Biotechnol. 2020, 62, 212–219. [Google Scholar] [CrossRef]
- Markl, E.; Grünbichler, H.; Lackner, M. Cyanobacteria for PHB Bioplastics Production: A Review. In Algae; Keung Wong, Y., Ed.; IntechOpen: London, UK, 2018; ISBN 9781838805623. [Google Scholar]
- Jim, P. OECD Policies for Bioplastics in the Context of a Bioeconomy. Ind. Biotechnol. 2014, 10, 19–21. [Google Scholar] [CrossRef]
- Doi, Y. Microbial Synthesis and Properties of Polyhydroxy-alkanoates. Mrs Bull. 1992, 17, 39–42. [Google Scholar] [CrossRef]
- Reis, M.A.M.; Serafim, L.S.; Lemos, P.C.; Ramos, A.M.; Aguiar, F.R.; Van Loosdrecht, M.C.M. Production of polyhydroxyalkanoates by mixed microbial cultures. Bioprocess. Biosyst. Eng. 2003, 25, 377–385. [Google Scholar] [CrossRef]
- Schlebusch, M.; Forchhammer, K. Requirement of the Nitrogen Starvation-Induced Protein Sll0783 for Polyhydroxybutyrate Accumulation in Synechocystis sp. Strain PCC 6803. AEM 2010, 76, 6101–6107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EN13432. Available online: https://www.beuth.de/de/norm/din-en-13432/32115376 (accessed on 11 July 2020).
- Rutkowska, M.; Heimowska, A.; Krasowska, K.; Janik, H. Biodegradability of Polyethylene Starch Blends in Sea Water. Pol. J. Environ. Stud. 2002, 11, 267–271. [Google Scholar]
- European Bioplastics What Types of Bioplastics Do Exist and What Properties Do They Have? Available online: https://www.european-bioplastics.org/faq-items/what-types-of-bioplastics-do-exist-and-what-properties-do-they-have/ (accessed on 24 June 2020).
- Storz, H.; Vorlop, K.-D. Bio-based plastics: Status, challenges and trends. Landbauforsch. Appl. Agric. Res. 2013, 321–332. [Google Scholar] [CrossRef]
- Bio-Plastic Packaging-Global Market Outlook (2016–2022). Available online: https://www.strategymrc.com/report/bio-plastic-packaging-market-2016 (accessed on 24 June 2020).
- European Bioplastics Market. Available online: https://www.european-bioplastics.org/market/ (accessed on 24 June 2020).
- Shrivastav, A.; Mishra, S.K.; Mishra, S. Polyhydroxyalkanoate (PHA) synthesis by Spirulina subsalsa from Gujarat coast of India. Int. J. Biol. Macromol. 2010, 46, 255–260. [Google Scholar] [CrossRef]
- Zinn, M.; Witholt, B.; Egli, T. Occurrence, synthesis and medical application of bacterial polyhydroxyalkanoate. Adv. Drug Deliv. Rev. 2001, 53, 5–21. [Google Scholar] [CrossRef]
- Chen, G.-Q.; Wu, Q. The application of polyhydroxyalkanoates as tissue engineering materials. Biomaterials 2005, 26, 6565–6578. [Google Scholar] [CrossRef]
- Gadgil, B.S.T.; Killi, N.; Rathna, G.V.N. Polyhydroxyalkanoates as biomaterials. Med. Chem. Commun. 2017, 8, 1774–1787. [Google Scholar] [CrossRef]
- Reddy, M.V.; Mohan, S.V. Polyhydroxy alkanoates Production by Newly Isolated Bacteria Serratia ureilytica Using Volatile Fatty Acids as Substrate: Bio-Electro Kinetic Analysis. J. Microb. Biochem. Technol. 2015, 7. [Google Scholar] [CrossRef]
- Chen, G.-Q. A microbial polyhydroxyalkanoates (PHA) based bio- and materials industry. Chem. Soc. Rev. 2009, 38, 2434. [Google Scholar] [CrossRef]
- Poli, A.; Di Donato, P.; Abbamondi, G.R.; Nicolaus, B. Synthesis, Production, and Biotechnological Applications of Exopolysaccharides and Polyhydroxyalkanoates by Archaea. Archaea 2011, 2011, 693253. [Google Scholar] [CrossRef] [Green Version]
- Anderson, A.J.; Dawes, E.A. Occurrence, metabolism, metabolic role, and industrial uses of bacterial polyhydroxyalkanoates. Microbiol. Rev. 1990, 54, 450–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalsasso, R.R.; Pavan, F.A.; Bordignon, S.E.; de Aragão, G.M.F.; Poletto, P. Polyhydroxybutyrate (PHB) production by Cupriavidus necator from sugarcane vinasse and molasses as mixed substrate. Process. Biochem. 2019, 85, 12–18. [Google Scholar] [CrossRef]
- Benesova, P.; Kucera, D.; Marova, I.; Obruca, S. Chicken feather hydrolysate as an inexpensive complex nitrogen source for PHA production by Cupriavidus necator on waste frying oils. Lett. Appl. Microbiol. 2017, 65, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Fidler, S.; Dennis, D. Polyhydroxyalkanoate production in recombinant Escherichia coli. Fems. Microbiol. Lett. 1992, 103, 231–235. [Google Scholar] [CrossRef]
- Wang, F.; Lee, S.Y. Production of poly(3-hydroxybutyrate) by fed-batch culture of filamentation-suppressed recombinant Escherichia coli. Appl. Environ. Microbiol. 1997, 63, 4765–4769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Lee, S.Y. High cell density culture of metabolically engineered Escherichia coli for the production of poly(3-hydroxybutyrate) in a defined medium. Biotechnol. Bioeng. 1998, 58, 325–328. [Google Scholar] [CrossRef]
- Weiner, R. Biopolymers from marine prokaryotes. Trends Biotechnol. 1997, 15, 390–394. [Google Scholar] [CrossRef]
- Macrae, R.M.; Wilkinson, J.F. Poly-hyroxybutyrate Metabolism in Washed Suspensions of Bacillus cereus and Bacillus megaterium. J. Gen. Microbiol. 1958, 19, 210–222. [Google Scholar] [CrossRef] [Green Version]
- Raza, Z.A.; Abid, S.; Banat, I.M. Polyhydroxyalkanoates: Characteristics, production, recent developments and applications. Int. Biodeterior. Biodegrad. 2018, 126, 45–56. [Google Scholar] [CrossRef]
- Westbrook, A.W.; Miscevic, D.; Kilpatrick, S.; Bruder, M.R.; Moo-Young, M.; Chou, C.P. Strain engineering for microbial production of value-added chemicals and fuels from glycerol. Biotechnol. Adv. 2019, 37, 538–568. [Google Scholar] [CrossRef]
- Singh, A.; Nigam, P.S.; Murphy, J.D. Renewable fuels from algae: An answer to debatable land based fuels. Bioresour. Technol. 2011, 102, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Halami, P.M. Production of polyhydroxyalkanoate from starch by the native isolate Bacillus cereus CFR06. World J. Microbiol. Biotechnol. 2008, 24, 805–812. [Google Scholar] [CrossRef]
- Markou, G.; Vandamme, D.; Muylaert, K. Microalgal and cyanobacterial cultivation: The supply of nutrients. Water Res. 2014, 65, 186–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Troschl, C.; Meixner, K.; Drosg, B. Cyanobacterial PHA Production—Review of Recent Advances and a Summary of Three Years’ Working Experience Running a Pilot Plant. Bioengineering 2017, 4, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tokiwa, Y.; Calabia, B.P. Review Degradation of microbial polyesters. Biotechnol. Lett. 2004, 26, 1181–1189. [Google Scholar] [CrossRef]
- Lemoigne, M. Products of dehydration and of polymerization of β-hydroxybutyric acid. Bull. Soc. Chem. Biol. 1926, 8, 770–782. [Google Scholar]
- Griffin, G.J.L. (Ed.) Chemistry and Technology of Biodegradable Polymers, 1st ed.; Blackie Academic & Professional: London, UK, 1994; ISBN 9780751400038. [Google Scholar]
- Lee, S.Y. Bacterial Polyb ydroxyalkanoates. Biotechnol. Bioeng. 1996, 49, 1–14. [Google Scholar] [CrossRef]
- Kunasundari, B.; Sudesh, K. Isolation and recovery of microbial polyhydroxyalkanoates. Express Polym. Lett. 2011, 5, 620–634. [Google Scholar] [CrossRef]
- Lee, S.Y.; Hong, S.H.; Park, S.J.; van Wegen, R.; Middelberg, A.P.J. Metabolic Flux Analysis on the Production of Poly(3-hydroxybutyrate) Biopolymers; Wiley: New York, NY, USA, 2005; pp. 249–257. [Google Scholar]
- Lim, S.-J.; Jung, Y.-M.; Shin, H.-D.; Lee, Y.-H. Amplification of the NADPH-related genes zwf and gnd for the oddball biosynthesis of PHB in an E. coli transformant harboring a cloned phbCAB operon. J. Biosci. Bioeng. 2002, 93, 543–549. [Google Scholar] [CrossRef]
- Fukui, T.; Doi, Y. Cloning and analysis of the poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) biosynthesis genes of Aeromonas caviae. J. Bact. 1997, 179, 4821–4830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuge, T.; Fukui, T.; Matsusaki, H.; Taguchi, S.; Kobayashi, G.; Ishizaki, A.; Doi, Y. Molecular cloning of two (R)-specific enoyl-CoA hydratase genes from Pseudomonas aeruginosa and their use for polyhydroxyalkanoate synthesis. FEMS Microbiol. Lett. 2000, 184, 193–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hein, J.; Paletta, A.; Steinbüchel, S. Cloning. Characterization and comparison of the Pseudomonas mendocina polyhydroxyalkanoate synthases PhaC1 and PhaC2. Appl. Microbiol. Biotechnol. 2002, 58, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Fukui, T.; Shiomi, N.; Doi, Y. Expression and characterization of (R)-specific enoyl coenzyme A hydratase involved in polyhydroxyalkanoate biosynthesis by Aeromonas caviae. J. Bacteriol. 1998, 180, 667–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kniewel, R.; Lopez, O.R.; Prieto, M.A. Biogenesis of medium-chain-length polyhydroxyalkanoates. In Biogenesis of Fatty Acids, Lipids and Membranes; Geiger, O., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 457–481. ISBN 9783319504292. [Google Scholar]
- Huijberts, G.N.; Eggink, G.; de Waard, P.; Huisman, G.W.; Witholt, B. Pseudomonas putida KT2442 cultivated on glucose accumulates poly(3-hydroxyalkanoates) consisting of saturated and unsaturated monomers. Appl. Environ. Microbiol. 1992, 58, 536–544. [Google Scholar] [CrossRef] [Green Version]
- Rehm, B.H.A.; Krüger, N.; Steinbüchel, A. A New Metabolic Link between Fatty Acid de Novo Synthesis and Polyhydroxyalkanoic Acid Synthesis: The phag gene from pseudomonas putida kt2440 encodes a 3-hydroxyacyl-acyl carrier protein-coenzyme a transferase. J. Biol. Chem. 1998, 273, 24044–24051. [Google Scholar] [CrossRef] [Green Version]
- Salehizadeh, H.; Van Loosdrecht, M.C.M. Production of polyhydroxyalkanoates by mixed culture: Recent trends and biotechnological importance. Biotechnol. Adv. 2004, 22, 261–279. [Google Scholar] [CrossRef]
- Wen, Q.; Chen, Z.; Tian, T.; Chen, W. Effects of phosphorus and nitrogen limitation on PHA production in activated sludge. J. Environ. Sci. 2010, 22, 1602–1607. [Google Scholar] [CrossRef]
- Rhu, D.H.; Lee, W.H.; Kim, J.Y.; Choi, E. Polyhydroxyalkanoate (PHA) production from waste. Water Sci. Technol. 2003, 48, 221–228. [Google Scholar] [CrossRef]
- Sureshkumar, M. Production of biodegradable plastics from activated sludge generated from a food processing industrial wastewater treatment plant. Bioresour. Technol. 2004, 95, 327–330. [Google Scholar] [CrossRef]
- Morgan-Sagastume, F.; Karlsson, A.; Johansson, P.; Pratt, S.; Boon, N.; Lant, P.; Werker, A. Production of polyhydroxyalkanoates in open, mixed cultures from a waste sludge stream containing high levels of soluble organics, nitrogen and phosphorus. Water Res. 2010, 44, 5196–5211. [Google Scholar] [CrossRef]
- Albuquerque, M.G.E.; Eiroa, M.; Torres, C.; Nunes, B.R.; Reis, M.A.M. Strategies for the development of a side stream process for polyhydroxyalkanoate (PHA) production from sugar cane molasses. J. Biotechnol. 2007, 130, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Montiel-Jarillo, G.; Carrera, J.; Suárez-Ojeda, M.E. Enrichment of a mixed microbial culture for polyhydroxyalkanoates production: Effect of pH and N and P concentrations. Sci. Total Environ. 2017, 583, 300–307. [Google Scholar] [CrossRef]
- Cui, Y.-W.; Gong, X.-Y.; Shi, Y.-P.; Wang, Z. (Drew) Salinity effect on production of PHA and EPS by Haloferax mediterranei. RSC Adv. 2017, 7, 53587–53595. [Google Scholar] [CrossRef] [Green Version]
- Albuquerque, M.G.E.; Torres, C.A.V.; Reis, M.A.M. Polyhydroxyalkanoate (PHA) production by a mixed microbial culture using sugar molasses: Effect of the influent substrate concentration on culture selection. Water Res. 2010, 44, 3419–3433. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, Y.; He, N.; Huang, J.; Zhu, K.; Shao, W.; Wang, H.; Yuan, W.; Li, Q. Optimization of polyhydroxybutyrate (PHB) production by excess activated sludge and microbial community analysis. J. Hazard. Mater. 2011, 185, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Panda, B.; Jain, P.; Sharma, L.; Mallick, N. Optimization of cultural and nutritional conditions for accumulation of poly-β-hydroxybutyrate in Synechocystis sp. PCC 6803. Bioresour. Technol. 2006, 97, 1296–1301. [Google Scholar] [CrossRef]
- Samantaray, S.; Nayak, J.K.; Mallick, N. Wastewater Utilization for Poly-β-Hydroxybutyrate Production by the Cyanobacterium Aulosira fertilissima in a Recirculatory Aquaculture System. Appl. Environ. Microbiol. 2011, 77, 8735–8743. [Google Scholar] [CrossRef] [Green Version]
- Dawes, E.A. Microbial energy reserve compounds. In Microbial Energetics; Blackie: Glasgow, UK, 1986; pp. 145–165. [Google Scholar]
- Tan, G.-Y.; Chen, C.-L.; Li, L.; Ge, L.; Wang, L.; Razaad, I.; Li, Y.; Zhao, L.; Mo, Y.; Wang, J.-Y. Start a Research on Biopolymer Polyhydroxyalkanoate (PHA): A Review. Polymers 2014, 6, 706–754. [Google Scholar] [CrossRef] [Green Version]
- Miller, N.D.; Williams, D.F. On the biodegradation of poly-β-hydroxybutyrate (PHB) homopolymer and poly-β-hydroxybutyrate-hydroxyvalerate copolymers. Biomaterials 1987, 8, 129–137. [Google Scholar] [CrossRef]
- Biazar, E.; Heidari Keshel, S. A nanofibrous PHBV tube with Schwann cell as artificial nerve graft contributing to Rat sciatic nerve regeneration across a 30-mm defect bridge. Cell Commun. Adhes. 2013, 20, 41–49. [Google Scholar] [CrossRef]
- Laycock, B.; Halley, P.; Pratt, S.; Werker, A.; Lant, P. The chemomechanical properties of microbial polyhydroxyalkanoates. Prog. Polym. Sci. 2013, 38, 536–583. [Google Scholar] [CrossRef]
- Kusaka, S.; Iwata, T.; Doi†*, Y. Microbial Synthesis and Physical Properties of Ultra-High-Molecular-Weight Poly[(R)-3-Hydroxybutyrate]. J. Macromol. Sci. Part A 1998, 35, 319–335. [Google Scholar] [CrossRef]
- Sudesh, K.; Abe, H.; Doi, Y. Synthesis, structure and properties of polyhydroxyalkanoates: Biological polyesters. Prog. Polym. Sci. 2000, 25, 1503–1555. [Google Scholar] [CrossRef]
- Kundu, P.P.; Nandy, A.; Mukherjee, A.; Pramanik, N. Polyhydroxyalkanoates: Microbial synthesis and applications. In Encyclopedia of Biomedical Polymers and Polymeric Biomaterials; CRC Press: Boca Raton, FL, USA, 2015; Volume 11, p. 10444. [Google Scholar]
- Bengtsson, S.; Pisco, A.R.; Reis, M.A.M.; Lemos, P.C. Production of polyhydroxyalkanoates from fermented sugar cane molasses by a mixed culture enriched in glycogen accumulating organisms. J. Biotechnol. 2010, 145, 253–263. [Google Scholar] [CrossRef]
- Bengtsson, S. The utilization of glycogen accumulating organisms for mixed culture production of polyhydroxyalkanoates. Biotechnol. Bioeng. 2009, 698–708. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zhang, G.; Park, S.; Lee, S. Industrial scale production of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate). Appl. Microbiol. Biotechnol. 2001, 57, 50–55. [Google Scholar] [CrossRef]
- Shimamura, E.; Kasuya, K.; Kobayashi, G.; Shiotani, T.; Shima, Y.; Doi, Y. Physical Properties and Biodegradability of Microbial Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate). Macromolecules 1994, 27, 878–880. [Google Scholar] [CrossRef]
- Battegazzore, D.; Noori, A.; Frache, A. Hemp hurd and alfalfa as particle filler to improve the thermo-mechanical and fire retardant properties of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate). Polym. Compos. 2019, 40, 3429–3437. [Google Scholar] [CrossRef]
- Urakami, T.; Imagawa, S.; Harada, M.; Iwamoto, A.; Tokiwa, Y. Development of Biodegradable Plastic-Poly-.BETA.-hydroxybutyrate/polycaprolaetone Blend Polymer. Kobunshi Ronbunshu 2000, 57, 263–270. [Google Scholar] [CrossRef] [Green Version]
- Savenkova, L.; Gercberga, Z.; Bibers, I.; Kalnin, M. Effect of 3-hydroxy valerate content on some physical and mechanical properties of polyhydroxyalkanoates produced by Azotobacter chroococcum. Process. Biochem. 2000, 36, 445–450. [Google Scholar] [CrossRef]
- Park, S.H.; Lee, S.H.; Lee, S.Y. Preparation of optically active β-amino acids from microbial polyester polyhydroxyalkanoates. J. Chem. Res. (S) 2001, 498–499. [Google Scholar] [CrossRef]
- Pederson, E.N.; McChalicher, C.W.J.; Srienc, F. Bacterial Synthesis of PHA Block Copolymers. Biomacromolecules 2006, 7, 1904–1911. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Tong, X.; Zhao, Y. Fast Photodegradable Block Copolymer Micelles for Burst Release. Macromolecules 2011, 44, 437–439. [Google Scholar] [CrossRef]
- Li, S.Y.; Dong, C.L.; Wang, S.Y.; Ye, H.M.; Chen, G.-Q. Microbial production of polyhydroxyalkanoate block copolymer by recombinant Pseudomonas putida. Appl. Microbiol. Biotechnol. 2011, 90, 659–669. [Google Scholar] [CrossRef]
- Clarinval, A.-M.; Halleux, J. 1-Classification of biodegradable polymers. In Biodegradable Polymers for Industrial Applications; Smith, R., Ed.; CRC Press: Boca Raton, FL, USA, 2005; pp. 3–56. [Google Scholar]
- Holmes, P.A. Biologically Produced (R)-3-Hydroxy- Alkanoate Polymers and Copolymers. In Developments in Crystalline Polymers; Bassett, D.C., Ed.; Springer: Dordrecht, The Netherlands, 1988; pp. 1–65. ISBN 9789401070966. [Google Scholar]
- Misra, S.K.; Valappil, S.P.; Roy, I.; Boccaccini, A.R. Polyhydroxyalkanoate (PHA)/Inorganic Phase Composites for Tissue Engineering Applications. Biomacromolecules 2006, 7, 2249–2258. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Zhang, Y.; Han, S.; Chen, Y.; Li, B.; Liao, M.; Chen, W.; Deng, X.; Zhao, J.; Huang, B. Synthesis and in vitro/in vivo anti-cancer evaluation of curcumin-loaded chitosan/poly(butyl cyanoacrylate) nanoparticles. Int. J. Pharm. 2010, 400, 211–220. [Google Scholar] [CrossRef]
- Valappil, S.P.; Boccaccini, A.R.; Bucke, C.; Roy, I. Polyhydroxyalkanoates in Gram-positive bacteria: Insights from the genera Bacillus and Streptomyces. Antonie Van Leeuwenhoek 2006, 91, 1–17. [Google Scholar] [CrossRef]
- Steinbüchel, A.; Füchtenbusch, B. Bacterial and other biological systems for polyester production. Trends Biotechnol. 1998, 16, 419–427. [Google Scholar] [CrossRef]
- Duvernoy, O.; Malm, T.; Ramström, J.; Bowald, S. A Biodegradable Patch used as a Pericardial Substitute after Cardiac Surgery: 6- and 24-Month Evaluation with CT. Thorac. Cardiovasc. Surg. 1995, 43, 271–274. [Google Scholar] [CrossRef]
- Atkins, T.W.; Peacock, S.J. The incorporation and release of bovine serum albumin from poly-hydroxybutyrate-hydroxyvalerate microcapsules. J. Microencapsul. 1996, 13, 709–717. [Google Scholar] [CrossRef]
- Tepha Patents. Available online: https://www.tepha.com/news-events/patents/ (accessed on 25 June 2020).
- Martin, D.P.; Williams, S.F. Medical applications of poly-4-hydroxybutyrate: A strong flexible absorbable biomaterial. Biochem. Eng. J. 2003, 16, 97–105. [Google Scholar] [CrossRef]
- Steinbüchel, A.; Valentin, H.E. Diversity of bacterial polyhydroxyalkanoic acids. FEMS Microbiol. Lett. 1995, 128, 219–228. [Google Scholar] [CrossRef]
- Seebach, D.; Herrmann, G.F.; Lengweiler, U.D.; Bachmann, B.M.; Amrein, W. Synthesis and Enzymatic Degradation of Dendrimers from(R)-3-Hydroxybutanoic Acid and Trimesic Acid. Angew. Chem. Int. Ed. Engl. 1996, 35, 2795–2797. [Google Scholar] [CrossRef]
- Bekker, A.; Holland, H.D.; Wang, P.-L.; Rumble, D.; Stein, H.J.; Hannah, J.L.; Coetzee, L.L.; Beukes, N.J. Dating the rise of atmospheric oxygen. Nature 2004, 427, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Olson, J.M. Photosynthesis in the Archean Era. Photosynth Res. 2006, 88, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Oren, A. Cyanobacteria: Biology, ecology and evolution. In Cyanobacteria; Sharma, N.K., Rai, A.K., Stal, L.J., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2013; pp. 1–20. ISBN 9781118402238. [Google Scholar]
- Kasting, J.F. EARTH HISTORY: The Rise of Atmospheric Oxygen. Science 2001, 293, 819–820. [Google Scholar] [CrossRef] [PubMed]
- Kasting, J.F. Life and the Evolution of Earth’s Atmosphere. Science 2002, 296, 1066–1068. [Google Scholar] [CrossRef] [Green Version]
- Cavalier-Smith, T. Chloroplast Evolution: Secondary Symbiogenesis and Multiple Losses. Curr. Biol. 2002, 12, R62–R64. [Google Scholar] [CrossRef] [Green Version]
- Stal, L.J. Physiological ecology of cyanobacteria in microbial mats and other communities. New Phytol. 1995, 131, 1–32. [Google Scholar] [CrossRef] [Green Version]
- Whitton, B.A.; Potts, M. The Ecology of Cyanobacteria: Their Diversity in Time and Space; Springer: Dordrecht, The Netherlands, 2002; ISBN 9780306468551. [Google Scholar]
- Waterbury, J.B.; Watson, S.W.; Valois, F.W.; Franks, D.G. Biological and ecological characterization of the marine unicellular cyanobacterium Synechococcus. In Photosynthetic Picoplankton; Platt, T., Li, W.K.W., Eds.; Canadian Bulletin of Fisheries and Aquatic Sciences: Ottawa, ON, Canada, 1986; Volume 214, pp. 71–120. ISBN 066012243X. [Google Scholar]
- Thajuddin, N.; Subramanian, G. Cyanobacterial biodiversity and potential applications in biotechnology. Curr. Sci. 2005, 89, 47–57. [Google Scholar]
- Lindell, D.; Padan, E.; Post, A.F. Regulation of ntcA Expression and Nitrite Uptake in the Marine Synechococcus sp. Strain WH 7803. J. Bacteriol. 1998, 180, 1878–1886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dodds, W.K.; Gudder, D.A.; Mollenhauer, D. The ecology of nostoc. J. Phycol. 1995, 31, 2–18. [Google Scholar] [CrossRef]
- Tsinoremas, N.F.; Castets, A.M.; Harrison, M.A.; Allen, J.F.; Tandeau de Marsac, N. Photosynthetic electron transport controls nitrogen assimilation in cyanobacteria by means of posttranslational modification of the glnB gene product. Proc. Natl. Acad. Sci. USA 1991, 88, 4565–4569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Latifi, A.; Ruiz, M.; Zhang, C.-C. Oxidative stress in cyanobacteria. FEMS Microbiol. Rev. 2009, 33, 258–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, Q.; Chen, Z.; Ge, F. Proteomic analysis of post translational modifications in cyanobacteria. J. Proteom. 2016, 134, 57–64. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, D.T.; da Costa, A.A.F.; Costa, F.F.; da Rocha Filho, G.N.; do Nascimento, L.A.S. Advances in the Biotechnological Potential of Brazilian Marine Microalgae and Cyanobacteria. Molecules 2020, 25, 2908. [Google Scholar] [CrossRef]
- Shimizu, Y. Microalgal metabolites. Curr. Opin. Microbiol. 2003, 6, 236–243. [Google Scholar] [CrossRef]
- Koehn, F.E.; Longley, R.E.; Reed, J.K. Microcolins A and B, New Immunosuppressive Peptides from the Blue-Green Alga Lyngbya majuscula. J. Nat. Prod. 1992, 55, 613–619. [Google Scholar] [CrossRef]
- Al-Batshan, H.A.; Al-Mufarrej, S.I.; Al-Homaidan, A.A.; Qureshi, M.A. Enhancement Of Chicken Macrophage Phagocytic Function And Nitrite Production By Dietary Spirulina platensis. Immunopharm. Immunotoxicol. 2001, 23, 281–289. [Google Scholar] [CrossRef]
- Qureshi, M.A.; Garlich, J.D.; Kidd, M.T. Dietary Spirulina Platensis Enhances Humoral and Cell-Mediated Immune Functions in Chickens. Immunopharmacol. Immunotoxicol. 1996, 18, 465–476. [Google Scholar] [CrossRef]
- Rajeev, K.J.; Xu, Z. Biomedical Compounds from Marine organisms. Mar. Drugs 2004, 2, 123–146. [Google Scholar] [CrossRef] [Green Version]
- Proksch, P.; Edrada, R.; Ebel, R. Drugs from the seas-current status and microbiological implications. Appl. Microbiol. Biotechnol. 2002, 59, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, W.W.; Gorham, P.R. The mosaic nature of toxic blooms of cyanobacteria. In The Water Environment; Carmichael, W.W., Ed.; Springer: Boston, MA, USA, 1981; pp. 161–172. ISBN 9781461332695. [Google Scholar]
- Belay, A.; Kato, T.; Ota, Y. Spirulina (Arthrospira): Potential application as an animal feed supplement. J. Appl. Phycol. 1996, 8, 303–311. [Google Scholar] [CrossRef]
- Ansari, S.; Fatma, T. Cyanobacterial Polyhydroxybutyrate (PHB): Screening, Optimization and Characterization. PLoS ONE 2016, 11, e0158168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beck, C.; Knoop, H.; Axmann, I.M.; Steuer, R. The diversity of cyanobacterial metabolism: Genome analysis of multiple phototrophic microorganisms. BMC Genom. 2012, 13, 56. [Google Scholar] [CrossRef] [Green Version]
- Aikawa, S.; Izumi, Y.; Matsuda, F.; Hasunuma, T.; Chang, J.-S.; Kondo, A. Synergistic enhancement of glycogen production in Arthrospira platensis by optimization of light intensity and nitrate supply. Bioresour. Technol. 2012, 108, 211–215. [Google Scholar] [CrossRef]
- Hu, Q.; Sommerfeld, M.; Jarvis, E.; Ghirardi, M.; Posewitz, M.; Seibert, M.; Darzins, A. Microalgal triacylglycerols as feedstocks for biofuel production: Perspectives and advances. Plant. J. 2008, 54, 621–639. [Google Scholar] [CrossRef]
- Damrow, R.; Maldener, I.; Zilliges, Y. The Multiple Functions of Common Microbial Carbon Polymers, Glycogen and PHB, during Stress Responses in the Non-Diazotrophic Cyanobacterium Synechocystis sp. PCC 6803. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef]
- De Jaeger, L.; Verbeek, R.E.; Draaisma, R.B.; Martens, D.E.; Springer, J.; Eggink, G.; Wijffels, R.H. Superior triacylglycerol (TAG) accumulation in starchless mutants of Scenedesmus obliquus: (I) mutant generation and characterization. Biotechnol. Biofuels 2014, 7, 69. [Google Scholar] [CrossRef]
- Bjornsson, W.J.; MacDougall, K.M.; Melanson, J.E.; O’Leary, S.J.B.; McGinn, P.J. Pilot-scale supercritical carbon dioxide extractions for the recovery of triacylglycerols from microalgae: A practical tool for algal biofuels research. J. Appl. Phycol. 2012, 24, 547–555. [Google Scholar] [CrossRef]
- Carr, N.G. The occurrence of poly-β-hydroxybutyrate in the blue-green alga, Chlorogloea fritschii. Biochim. Et Biophys. Acta (Bba) Biophys. Incl. Photosynth. 1966, 120, 308–310. [Google Scholar] [CrossRef]
- Khetkorn, W.; Incharoensakdi, A.; Lindblad, P.; Jantaro, S. Enhancement of poly-3-hydroxybutyrate production in Synechocystis sp. PCC 6803 by overexpression of its native biosynthetic genes. Bioresour. Technol. 2016, 214, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Kamravamanesh, D.; Pflügl, S.; Nischkauer, W.; Limbeck, A.; Lackner, M.; Herwig, C. Photosynthetic poly-β-hydroxybutyrate accumulation in unicellular cyanobacterium Synechocystis sp. PCC 6714. AMB Expr. 2017, 7, 143. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, M.; Nakai, K.; Miyake, M.; Asada, Y.; Taya, M. Production of poly-β-hydroxybutyrate by thermophilic cyanobacterium, Synechococcus sp. MA19, under phosphate-limited conditions. Biotechnol. Lett. 2001, 23, 1095–1099. [Google Scholar] [CrossRef]
- Bhati, R.; Mallick, N. Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) copolymer production by the diazotrophic cyanobacterium Nostoc muscorum Agardh: Process optimization and polymer characterization. Algal Res. 2015, 7, 78–85. [Google Scholar] [CrossRef]
- Samantaray, S.; Mallick, N. Production and characterization of poly-β-hydroxybutyrate (PHB) polymer from Aulosira fertilissima. J. Appl. Phycol. 2012, 24, 803–814. [Google Scholar] [CrossRef]
- Hein, S.; Tran, H.; Steinbüchel, A. Synechocystis sp. PCC6803 possesses a two-component polyhydroxyalkanoic acid synthase similar to that of anoxygenic purple sulfur bacteria. Arch. Microbiol. 1998, 170, 162–170. [Google Scholar] [CrossRef]
- Matsusaki, H.; Manji, S.; Taguchi, K.; Kato, M.; Fukui, T.; Doi, Y. Cloning and molecular analysis of the Poly(3-hydroxybutyrate) and Poly(3-hydroxybutyrate-co-3-hydroxyalkanoate) biosynthesis genes in Pseudomonas sp. strain 61-3. J. Bacteriol. 1998, 180, 6459–6467. [Google Scholar] [CrossRef] [Green Version]
- Lane, C.E.; Benton, M.G. Detection of the enzymatically-active polyhydroxyalkanoate synthase subunit gene, phaC, in cyanobacteria via colony PCR. Mol. Cell. Probes 2015, 29, 454–460. [Google Scholar] [CrossRef]
- Erb, T.J.; Zarzycki, J. Biochemical and synthetic biology approaches to improve photosynthetic CO2-fixation. Curr. Opin. Chem. Biol. 2016, 34, 72–79. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, K.; Saito, J.; Yokoo, T.; Hori, C.; Nagata, A.; Kudoh, Y.; Ooi, T.; Taguchi, S. Ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO)-mediated de novo synthesis of glycolate-based polyhydroxyalkanoate in Escherichia coli. J. Biosci. Bioeng. 2019, 128, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Abo, B.O.; Odey, E.A.; Bakayoko, M.; Kalakodio, L. Microalgae to biofuels production: A review on cultivation, application and renewable energy. Rev. Environ. Health 2019, 34, 91–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marinho-Soriano, E.; Panucci, R.A.; Carneiro, M.A.A.; Pereira, D.C. Evaluation of Gracilaria caudata J. Agardh for bioremediation of nutrients from shrimp farming wastewater. Bioresour. Technol. 2009, 100, 6192–6198. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Raouf, N.; Al-Homaidan, A.A.; Ibraheem, I.B.M. Microalgae and wastewater treatment. Saudi J. Biol. Sci. 2012, 19, 257–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.; Srivastava, J.K.; Mallick, N.; Singh, A.K. Commercialization of Bacterial Cell Factories for the Sustainable Production of Polyhydroxyalkanoate Thermoplastics: Progress and Prospects. Recent Pat. Biotechnol. 2015, 9, 4–21. [Google Scholar] [CrossRef] [PubMed]
- Pouliot, Y.; Buelna, G.; Racine, C.; de la Noüe, J. Culture of cyanobacteria for tertiary wastewater treatment and biomass production. Biol. Wastes 1989, 29, 81–91. [Google Scholar] [CrossRef]
- Oswald, W.J. Micro-algae and wastewater treatment. In Micro-Algal Biotechnology; Borowitzka, M.A., Borowitzka, L.J., Eds.; Cambridge University Press: Cambridge, UK, 1988; pp. 305–328. [Google Scholar]
- Theregowda, R.B.; Vidic, R.; Landis, A.E.; Dzombak, D.A.; Matthews, H.S. Integrating external costs with life cycle costs of emissions from tertiary treatment of municipal wastewater for reuse in cooling systems. J. Clean. Prod. 2016, 112, 4733–4740. [Google Scholar] [CrossRef] [Green Version]
- Sood, A.; Renuka, N.; Prasanna, R.; Ahluwalia, A.S. Cyanobacteria as Potential Options for Wastewater Treatment. In Phytoremediation; Ansari, A.A., Gill, S.S., Gill, R., Lanza, G.R., Newman, L., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 83–93. ISBN 9783319109688. [Google Scholar]
- Su, Y.; Mennerich, A.; Urban, B. Comparison of nutrient removal capacity and biomass settleability of four high-potential microalgal species. Bioresour. Technol. 2012, 124, 157–162. [Google Scholar] [CrossRef]
- Cañizares-Villanueva, R.O.; Ramos, A.; Corona, A.I.; Monroy, O.; de la Torre, M.; Gomez-Lojero, C.; Travieso, L. Phormidium treatment of anaerobically treated swine wastewater. Water Res. 1994, 28, 1891–1895. [Google Scholar] [CrossRef]
- Chevalier, P.; Proulx, D.; Lessard, P.; Vincent, W.F.; de la Noüe, J. Nitrogen and phosphorus removal by high latitude mat-forming cyanobacteria for potential use in tertiary wastewater treatment. J. Appl. Phycol. 2000, 12, 105–112. [Google Scholar] [CrossRef]
- Talbot, P.; de la Noüe, J. Tertiary treatment of wastewater with Phormidium bohneri (Schmidle) under various light and temperature conditions. Water Res. 1993, 27, 153–159. [Google Scholar] [CrossRef]
- Honda, R.; Boonnorat, J.; Chiemchaisri, C.; Chiemchaisri, W.; Yamamoto, K. Carbon dioxide capture and nutrients removal utilizing treated sewage by concentrated microalgae cultivation in a membrane photobioreactor. Bioresour. Technol. 2012, 125, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Arias, D.M.; Uggetti, E.; García-Galán, M.J.; García, J. Cultivation and selection of cyanobacteria in a closed photobioreactor used for secondary effluent and digestate treatment. Sci. Total Environ. 2017, 587–588, 157–167. [Google Scholar] [CrossRef]
- Md Din, M.F.; Ujang, Z.; van Loosdrecht, M.C.M.; Ahmad, A.; Sairan, M.F. Optimization of nitrogen and phosphorus limitation for better biodegradable plastic production and organic removal using single fed-batch mixed cultures and renewable resources. Water Sci. Technol. 2006, 53, 15–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srebotnjak, T.; Carr, G.; de Sherbinin, A.; Rickwood, C. A global Water Quality Index and hot-deck imputation of missing data. Ecol. Indic. 2012, 17, 108–119. [Google Scholar] [CrossRef]
- Bhati, R.; Mallick, N. Carbon dioxide and poultry waste utilization for production of polyhydroxyalkanoate biopolymers by Nostoc muscorum Agardh: A sustainable approach. J. Appl. Phycol. 2016, 28, 161–168. [Google Scholar] [CrossRef]
- Liebal, U.W.; Blank, L.M.; Ebert, B.E. CO2 to succinic acid—Estimating the potential of biocatalytic routes. Metab. Eng. Commun. 2018, 7, e00075. [Google Scholar] [CrossRef]
- Wang, B.; Li, Y.; Wu, N.; Lan, C.Q. CO2 bio-mitigation using microalgae. Appl. Microbiol. Biotechnol. 2008, 79, 707–718. [Google Scholar] [CrossRef]
- Olguín, E.J.; Galicia, S.; Mercado, G.; Pérez, T. Annual productivity of Spirulina (Arthrospira) and nutrient removal in a pig wastewater recycling process under tropical conditions. J. Appl. Phycol. 2003, 15, 249–257. [Google Scholar] [CrossRef]
- Sweeten, J.M. Livestock and poultry waste management: A national overview. In National Livestock, Poultry, And Aquaculture Waste Management; American Society of Agricultural Engineers: San Jose, MI, USA, 1992; p. 414. [Google Scholar]
- Chaiklahan, R.; Chirasuwan, N.; Siangdung, W.; Paithoonrangsarid, K.; Bunnag, B. Cultivation of Spirulina platensis Using Pig Wastewater in a Semi-Continuous Process. J. Microbiol. Biotechnol. 2010, 20, 609–614. [Google Scholar] [CrossRef] [Green Version]
- Zinn, M.; Amstutz, V.; Hanik, N.; Pott, J.; Utsunomia, C. Grave-to-cradle: The potential of autotrophic bioprocesses in bioplastic production. New Biotechnol. 2018, 44, S64. [Google Scholar] [CrossRef]
- Morillo, J.A.; Antizar-Ladislao, B.; Monteoliva-Sánchez, M.; Ramos-Cormenzana, A.; Russell, N.J. Bioremediation and biovalorisation of olive-mill wastes. Appl. Microbiol. Biotechnol. 2009, 82, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, J.; Honkasalo, A.; Seppälä, J. Circular Economy: The Concept and its Limitations. Ecol. Econ. 2018, 143, 37–46. [Google Scholar] [CrossRef]
- Wang, L.; Min, M.; Li, Y.; Chen, P.; Chen, Y.; Liu, Y.; Wang, Y.; Ruan, R. Cultivation of Green Algae Chlorella sp. in Different Wastewaters from Municipal Wastewater Treatment Plant. Appl. Biochem. Biotechnol. 2010, 162, 1174–1186. [Google Scholar] [CrossRef] [PubMed]
- Chew, K.W.; Yap, J.Y.; Show, P.L.; Suan, N.H.; Juan, J.C.; Ling, T.C.; Lee, D.-J.; Chang, J.-S. Microalgae biorefinery: High value products perspectives. Bioresour. Technol. 2017, 229, 53–62. [Google Scholar] [CrossRef]
- Haas, R.; Jin, B.; Zepf, F.T. Production of Poly(3-hydroxybutyrate) from Waste Potato Starch. Biosci. Biotechnol. Biochem. 2008, 72, 253–256. [Google Scholar] [CrossRef]
- Cuellar-Bermudez, S.P.; Garcia-Perez, J.S.; Rittmann, B.E.; Parra-Saldivar, R. Photosynthetic bioenergy utilizing CO2: An approach on flue gases utilization for third generation biofuels. J. Clean. Prod. 2015, 98, 53–65. [Google Scholar] [CrossRef] [Green Version]
- Meixner, K.; Kovalcik, A.; Sykacek, E.; Gruber-Brunhumer, M.; Zeilinger, W.; Markl, K.; Haas, C.; Fritz, I.; Mundigler, N.; Stelzer, F.; et al. Cyanobacteria Biorefinery—Production of poly(3-hydroxybutyrate) with Synechocystis salina and utilisation of residual biomass. J. Biotechnol. 2018, 265, 46–53. [Google Scholar] [CrossRef]
- Directive 2002/32/EC of the European Parliament and of the Council of 7 May 2002 on Undesirable Substances in Animal Feed-COUNCIL Statement. Available online: https://eur-lex.europa.eu/eli/dir/2002/32/2015-02-27 (accessed on 25 June 2020).
- Gradíssimo, D.G.; Mourão, M.M.; Santos, A.V. Importância do Monitoramento de Cianobactérias e Suas Toxinas em Águas Para Consumo Humano. J. Crim. 2020, 9, 15–21. [Google Scholar] [CrossRef]
- Price, S.; Kuzhiumparambil, U.; Pernice, M.; Ralph, P.J. Cyanobacterial polyhydroxybutyrate for sustainable bioplastic production: Critical review and perspectives. J. Environ. Chem. Eng. 2020, 8, 104007. [Google Scholar] [CrossRef]
- Chaiklahan, R.; Chirasuwan, N.; Triratana, P.; Loha, V.; Tia, S.; Bunnag, B. Polysaccharide extraction from Spirulina sp. and its antioxidant capacity. Int. J. Biol. Macromol. 2013, 58, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Gomes, I.G.; Chaves, F.H.; Barros, R.N.; Moreira, R.L.; Teixeira, E.G.; Moreira, A.G.; Farias, W.R. Dietary supplementation with Spirulina platensis increases growth and color of red tilapia. Rev. Colomb. Ciencias Pecu 2012, 25, 462–471. [Google Scholar]
- Zahroojian, N.; Moravej, H.; Shivazad, M. Effects of Dietary Marine Algae (Spirulina platensis) on Egg Quality and Production Performance of Laying Hens. J. Agric. Sci. Technol. 2013, 15, 1353–1360. [Google Scholar]
- Briens, C.; Piskorz, J.; Berruti, F. Biomass Valorization for Fuel and Chemicals Production—A Review. Int. J. Chem. React. Eng. 2008, 6. [Google Scholar] [CrossRef]
- De Godos, I.; Blanco, S.; García-Encina, P.A.; Becares, E.; Muñoz, R. Long-term operation of high rate algal ponds for the bioremediation of piggery wastewaters at high loading rates. Bioresour. Technol. 2009, 100, 4332–4339. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, L.; Subramanian, G.; Nazeer, T.T.; Simpson, H.S.; Rahuman, S.T.; Raju, P. Cyanobacteria cultivation in industrial wastewaters and biodiesel production from their biomass: A review. Biotechnol. Appl. Biochem. 2011, 58, 220–225. [Google Scholar] [CrossRef]
- Sarsekeyeva, F.; Zayadan, B.K.; Usserbaeva, A.; Bedbenov, V.S.; Sinetova, M.A.; Los, D.A. Cyanofuels: Biofuels from cyanobacteria. Reality and perspectives. Photosynth. Res. 2015, 125, 329–340. [Google Scholar] [CrossRef]
- Lima, A.R.J.; Siqueira, A.S.; dos Santos, B.G.S.; da Silva, F.D.F.; Lima, C.P.; Cardoso, J.F.; Vianez Junior, J.L.d.S.G.; Dall’Agnol, L.T.; McCulloch, J.A.; Nunes, M.R.T.; et al. Draft Genome Sequence of the Brazilian Cyanobium sp. Strain CACIAM 14. Genome Announc. 2014, 2, e00669-14. [Google Scholar] [CrossRef] [Green Version]
- Lima, A.R.J.; de Castro, W.O.; Moraes, P.H.G.; Siqueira, A.S.; Aguiar, D.C.F.; de Lima, C.P.S.; Vianez-Júnior, J.L.S.G.; Nunes, M.R.T.; Dall’Agnol, L.T.; Gonçalves, E.C. Draft Genome Sequence of Alkalinema sp. Strain CACIAM 70d, a Cyanobacterium Isolated from an Amazonian Freshwater Environment. Genome Announc. 2017, 5, e00635-17. [Google Scholar] [CrossRef] [Green Version]
- Aboim, J.B.; Oliveira, D.; Ferreira, J.E.; Siqueira, A.S.; Dall’Agnol, L.T.; Rocha Filho, G.N.; Gonçalves, E.C.; Nascimento, L.A. Determination of biodiesel properties based on a fatty acid profile of eight Amazon cyanobacterial strains grown in two different culture media. RSC Adv. 2016, 6, 109751–109758. [Google Scholar] [CrossRef]
- de Oliveira, D.T.; Turbay Vasconcelos, C.; Feitosa, A.M.T.; Aboim, J.B.; de Oliveira, A.N.; Xavier, L.P.; Santos, A.S.; Gonçalves, E.C.; da Rocha Filho, G.N.; do Nascimento, L.A.S. Lipid profile analysis of three new Amazonian cyanobacteria as potential sources of biodiesel. Fuel 2018, 234, 785–788. [Google Scholar] [CrossRef]
- Gradíssimo, D.G.; Mourão, M.M.; do Amaral, S.C.; Lima, A.R.J.; Gonçalves, E.C.; Xavier, L.P.; Santos, A.V. Potencial produção de biomaterial pela cianobactéria amazônica Tolypothrix SP. CACIAM 22. In A Produção do Conhecimento nas Ciências da Saúde; Atena Editora: Belo Horizonte, Brazil, 2019; pp. 213–224. ISBN 9788572472982. [Google Scholar]



| Cyanobacteria | Mode | Nutritional Deprivation | Nutritional Supplementation | PHA | Production % (dcw) | Reference |
|---|---|---|---|---|---|---|
| Synechocystis sp. PCC6803 | Mixotrophic | P | Acetate | PHB | 28.8 | [77] |
| Synechocystis sp. PCC6803 | Mixotrophic | N | Acetate | PHB | 14.6 | [77] |
| Synechocystis sp. PCC6803 (mutant) | Mixotrophic | - | Acetate | PHB | 35 | [142] |
| Synechocystis sp. PCC6803 | Photoautotrophic | N, P | - | PHB | 16.4 | [143] |
| Synechococcus sp. MA19 | Photoautotrophic | P | - | PHB | 55 | [144] |
| Nostoc muscorum Agardh | Mixotrophic | N | Glucose, acetate, valerate | PHBV | 78 | [145] |
| Chlorogloea fritschii | Mixotrophic | - | Acetate | PHB | 10 | [141] |
| Spirulina subsalsa | Photoautotrophic | N | - | PHB | 14.7 | [32] |
| Aulosira fertilissima | Mixotrophic | N, P | Acetate, citrate | PHB | 85 | [146] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes Gradíssimo, D.; Pereira Xavier, L.; Valadares Santos, A. Cyanobacterial Polyhydroxyalkanoates: A Sustainable Alternative in Circular Economy. Molecules 2020, 25, 4331. https://doi.org/10.3390/molecules25184331
Gomes Gradíssimo D, Pereira Xavier L, Valadares Santos A. Cyanobacterial Polyhydroxyalkanoates: A Sustainable Alternative in Circular Economy. Molecules. 2020; 25(18):4331. https://doi.org/10.3390/molecules25184331
Chicago/Turabian StyleGomes Gradíssimo, Diana, Luciana Pereira Xavier, and Agenor Valadares Santos. 2020. "Cyanobacterial Polyhydroxyalkanoates: A Sustainable Alternative in Circular Economy" Molecules 25, no. 18: 4331. https://doi.org/10.3390/molecules25184331
APA StyleGomes Gradíssimo, D., Pereira Xavier, L., & Valadares Santos, A. (2020). Cyanobacterial Polyhydroxyalkanoates: A Sustainable Alternative in Circular Economy. Molecules, 25(18), 4331. https://doi.org/10.3390/molecules25184331