Antifungal Styryloquinolines as Candida albicans Efflux Pump Inhibitors: Styryloquinolines are ABC Transporter Inhibitors
Abstract
:1. Introduction
2. Results
2.1. Antifungal Activity
2.2. The Rhodamine 6G Efflux Assay
2.3. The Influence of Styrylquinolines on the Cdr1p Level
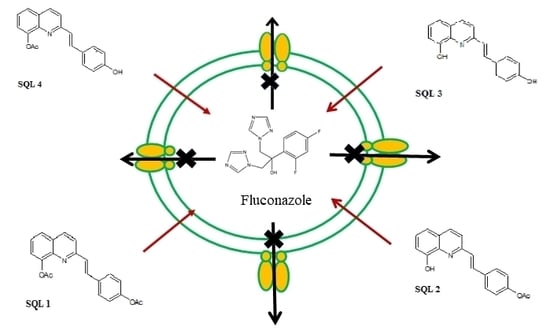
3. Discussion
4. Materials and Methods
4.1. Compounds
4.2. Strains and Growth Media
4.3. Susceptibility Testing
4.4. Permeabilization Assays
4.5. Western Blotting
4.6. Rhodamine 6G Assay
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Perlroth, J.; Choi, B.; Spellberg, B. Nosocomial fungal infections: Epidemiology, diagnosis, and treatment. Med. Mycol. 2007, 45, 321–346. [Google Scholar] [CrossRef]
- Akins, R.A. An update on antifungal targets and mechanisms of resistance in Candida albicans. Med. Mycol. 2005, 43, 285–318. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, F.; Gershkovich, P.; Sivak, O.; Wasan, E.K.; Wasan, K.M. Pharmacokinetics and tissue distribution of amphotericin B following oral administration of three lipid-based formulations to rats. Drug Dev. Ind. Pharm. 2013, 39, 1277–1283. [Google Scholar] [CrossRef]
- Gruszecki, W.I.; Gagos, M.; Kernen, P. Polyene antibiotic amphotericin B in monomolecular layers: Spectrophotometric and scanning force microscopic analysis. FEBS Lett. 2002, 524, 92–96. [Google Scholar] [CrossRef] [Green Version]
- Odds, F. Antifungal agents: Mechanisms of action. Trends Microbiol. 2003, 11, 272–279. [Google Scholar] [CrossRef]
- Suchodolski, J.; Muraszko, J.; Bernat, P.; Krasowska, A. A Crucial Role for Ergosterol in Plasma Membrane Composition, Localisation, and Activity of Cdr1p and H+-ATPase in Candida albicans. Microorganisms 2019, 7, 378. [Google Scholar] [CrossRef] [Green Version]
- Prasad, R.; Rawal, M.K. Efflux pump proteins in antifungal resistance. Front. Pharmacol. 2014, 5, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Mrozek-Wilczkiewicz, A.; Kalinowski, D.S.; Musiol, R.; Finster, J.; Szurko, A.; Serafin, K.; Knas, M.; Kamalapuram, S.K.; Kovacevic, Z.; Jampilek, J.; et al. Investigating the anti-proliferative activity of styrylazanaphthalenes and azanaphthalenediones. Bioorg. Med. Chem. 2010, 18, 2664–2671. [Google Scholar] [CrossRef]
- Mrozek-Wilczkiewicz, A.; Kuczak, M.; Malarz, K.; Cieślik, W.; Spaczyńska, E.; Musiol, R. The synthesis and anticancer activity of 2-styrylquinoline derivatives. A p53 independent mechanism of action. Eur. J. Med. Chem. 2019, 177, 338–349. [Google Scholar] [CrossRef]
- Cieslik, W.; Musiol, R.; Nycz, J.E.; Jampilek, J.; Vejsova, M.; Wolff, M.; Machura, B.; Polanski, J. Contribution to investigation of antimicrobial activity of styrylquinolines. Bioorg. Med. Chem. 2012, 20, 6960–6968. [Google Scholar] [CrossRef]
- Cieslik, W.; Spaczynska, E.; Malarz, K.; Tabak, D.; Nevin, E.; O’Mahony, J.; Coffey, A.; Mrozek-Wilczkiewicz, A.; Jampilek, J.; Musiol, R. Investigation of the Antimycobacterial Activity of 8-Hydroxyquinolines. Med. Chem. 2015, 11, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Musiol, R.; Serda, M.; Hensel-Bielowka, S.; Polanski, J. Quinoline-based antifungals. Curr. Med. Chem. 2010, 17, 1960–1973. [Google Scholar] [CrossRef] [PubMed]
- Musiol, R.; Jampilek, J.; Buchta, V.; Silva, L.; Niedbala, H.; Podeszwa, B.; Palka, A.; Majerz-Maniecka, K.; Oleksyn, B.; Polanski, J. Antifungal properties of new series of quinoline derivatives. Bioorg. Med. Chem. 2006, 14, 3592–3598. [Google Scholar] [CrossRef] [PubMed]
- Szczepaniak, J.; Cieślik, W.; Romanowicz, A.; Musioł, R.; Krasowska, A. Blocking and dislocation of Candida albicans Cdr1p transporter by styrylquinolines. Int. J. Antimicrob. Agents 2017, 50, 171–176. [Google Scholar] [CrossRef]
- Gershon, H.; Clarke, D.D.; Gershon, M. Synergistic Antifungal Action of 8-Quinolinol and Its Bischelate with Copper(Ⅱ) and with Mixed Ligand Chelates Composed of Copper(Ⅱ), 8-Quinolinol, and Aromatic Hydroxy Acids. J. Pharm. Sci. 1989, 78, 975–978. [Google Scholar] [CrossRef]
- Gershon, H.; Clarke, D.D.; Gershon, M. Evidence of Steric Factors in the Fungitoxic Mechanisms of 8-Quinolinol and Its 5- and 7-Halogenated Analogues. J. Pharm. Sci. 1991, 80, 542–544. [Google Scholar] [CrossRef]
- Gershon, H.; Gershon, M. Intramolecular synergism, an explanation for the enhanced fungitoxicity of halo-8-quinolinols. Mon. Chem. Chem. Mon. 1995, 126, 1303–1309. [Google Scholar] [CrossRef]
- Gershon, H.; Clarke, D.D.; McMahon, J.J.; Gershon, M. Preparation and Fungitoxicity of Some Dibromo-8-quinolinols. Mon. Chem. Chem. Mon. 2001, 132, 833–838. [Google Scholar] [CrossRef]
- Urbina, J.M.; Cortes, J.C.G.; Palma, A.; Lopez, S.N.; Zacchino, S.A.; Enriz, R.D.; Ribas, J.C.; Kouznetsov, V.V. Inhibitors of the fungal cell wall. Synthesis of 4-aryl-4-N-arylamine-1-butenes and related compounds with inhibitory activities on β(1–3) glucan and chitin synthases. Bioorg. Med. Chem. 2000, 8, 691–698. [Google Scholar] [CrossRef]
- Ghorab, M.; Abdel-Hamide, S.; Farrag, H. Synthesis of novel quinolines, pyranoquinolines, furoquinolines, thieno-quinoline and their effect on the ultrastructure of some pathogenic microorganisms. Acta Pol Pharm. 2001, 58, 175–184. [Google Scholar]
- Vandekerckhove, S.; Tran, H.G.; Desmet, T.; D’hooghe, M. Evaluation of (4-aminobutyloxy)quinolines as a novel class of antifungal agents. Bioorg. Med. Chem. Lett. 2013, 23, 4641–4643. [Google Scholar] [CrossRef]
- Hazra, A.; Mondal, S.; Maity, A.; Naskar, S.; Saha, P.; Paira, R.; Sahu, K.B.; Paira, P.; Ghosh, S.; Sinha, C.; et al. Amberlite-IRA-402 (OH) ion exchange resin mediated synthesis of indolizines, pyrrolo [1,2-a] quinolines and isoquinolines: Antibacterial and antifungal evaluation of the products. Eur. J. Med. Chem. 2011, 46, 2132–2140. [Google Scholar] [CrossRef]
- Delattin, N.; Bardiot, D.; Marchand, A.; Chaltin, P.; De Brucker, K.; Cammue, B.P.A.; Thevissen, K. Identification of fungicidal 2,6-disubstituted quinolines with activity against candida biofilms. Molecules 2012, 17, 12243–12251. [Google Scholar] [CrossRef] [Green Version]
- Mrozek-Wilczkiewicz, A.; Spaczynska, E.; Malarz, K.; Cieslik, W.; Rams-Baron, M.; Kryštof, V.; Musiol, R. Design, Synthesis and In Vitro Activity of Anticancer Styrylquinolines The p53 Independent Mechanism of Action. PLoS ONE 2015, 10, e0142678. [Google Scholar] [CrossRef] [Green Version]
- Mekouar, K.; Mouscadet, J.F.; Desmaële, D.; Subra, F.; Leh, H.; Savouré, D.; Auclair, C.; d’Angelo, J. Styrylquinoline derivatives: A new class of potent HIV-1 integrase inhibitors that block HIV-1 replication in CEM cells. J. Med. Chem. 1998, 41, 2846–2857. [Google Scholar] [CrossRef]
- Zouhiri, F.; Danet, M.; Bénard, C.; Normand-Bayle, M.; Mouscadet, J.-F.; Leh, H.; Marie Thomas, C.; Mbemba, G.; D’Angelo, J.; Desmaële, D. HIV-1 replication inhibitors of the styrylquinoline class: Introduction of an additional carboxyl group at the C-5 position of the quinoline. Tetrahedron Lett. 2005, 46, 2201–2205. [Google Scholar] [CrossRef]
- Zouhiri, F.; Mouscadet, J.F.; Mekouar, K.; Desmaële, D.; Savouré, D.; Leh, H.; Subra, F.; Le Bret, M.; Auclair, C.; d’Angelo, J. Structure-activity relationships and binding mode of styrylquinolines as potent inhibitors of HIV-1 integrase and replication of HIV-1 in cell culture. J. Med. Chem. 2000, 43, 1533–1540. [Google Scholar] [CrossRef]
- Machura, B.; Wolff, M.; Kowalczyk, W.; Musiol, R. Novel rhenium(V) complexes of 8-hydroxyquinoline derivatives—Synthesis, spectroscopic characterization, X-ray structure and DFT calculations. Polyhedron 2012, 33, 388–395. [Google Scholar] [CrossRef]
- Machura, B.; Wolff, M.; Cieślik, W.; Musioł, R. Novel oxorhenium(V) complexes of 8-hydroxyquinoline derivatives—Synthesis, spectroscopic characterization, X-ray crystal structures and DFT calculations. Polyhedron 2013, 51, 263–274. [Google Scholar] [CrossRef]
- Musiol, R. Styrylquinoline—A versatile scaffold in medicinal chemistry. Med. Chem. 2019, 15. [Google Scholar] [CrossRef]
- Musiol, R.; Podeszwa, B.; Finster, J.; Niedbala, H.; Polanski, J. An Efficient Microwave-Assisted Synthesis of Structurally Diverse Styrylquinolines. Mon. Chem. Chem. Mon. 2006, 137, 1211–1217. [Google Scholar] [CrossRef]
- Musiol, R.; Mrozek-Wilczkiewicz, A.; Polanski, J. Synergy against fungal pathogens: Working together is better than working alone. Curr. Med. Chem. 2014, 21, 870–893. [Google Scholar] [CrossRef]
- Henry, K.W.; Cruz, M.C.; Katiyar, S.K.; Edlind, T.D. Antagonism of azole activity against Candida albicans following induction of multidrug resistance genes by selected antimicrobial agents. Antimicrob. Agents Chemother. 1999, 43, 1968–1974. [Google Scholar] [CrossRef] [Green Version]
- Szczepaniak, J.; Łukaszewicz, M.; Krasowska, A. Estimation of Candida albicans ABC Transporter Behavior in Real-Time via Fluorescence. Front. Microbiol. 2015, 6, 1382. [Google Scholar] [CrossRef] [Green Version]
- Hibbert, F.; Spiers, K.J. Kinetics of hydrolysis of 1-Acetoxy-, 1-Acetoxy-8-hydroxy-, and 1,8-diacetoxy-naphthalenes; intermolecular participation by a hydroxy group. J. Chem. Soc. Perkin Trans. 1988, 20, 571–574. [Google Scholar] [CrossRef]
- Cipiciani, A.; Fringuelli, F.; Scappini, A.M. Enzymatic hydrolyses of acetoxy- and phenethylbenzoates by Candida cylindracea lipase. Tetrahedron 1996, 52, 9869–9876. [Google Scholar] [CrossRef]
- Rams-Baron, M.; Dulski, M.; Mrozek-Wilczkiewicz, A.; Korzec, M.; Cieslik, W.; Spaczyńska, E.; Bartczak, P.; Ratuszna, A.; Polanski, J.; Musiol, R. Synthesis of New Styrylquinoline Cellular Dyes, Fluorescent Properties, Cellular Localization and Cytotoxic Behavior. PLoS ONE 2015, 10, e0131210. [Google Scholar] [CrossRef]
- Musiol, R.; Jampilek, J.; Nycz, J.E.; Pesko, M.; Carroll, J.; Kralova, K.; Vejsova, M.; O’Mahony, J.; Coffey, A.; Mrozek, A.; et al. Investigating the activity spectrum for ring-substituted 8-hydroxyquinolines. Molecules 2010, 15, 288–304. [Google Scholar] [CrossRef] [Green Version]
- Evensen, N.A.; Braun, P.C. The effects of tea polyphenols on Candida albicans: Inhibition of biofilm formation and proteasome inactivation. Can. J. Microbiol. 2009, 55, 1033–1039. [Google Scholar] [CrossRef]
- Shahzad, M.; Sherry, L.; Rajendran, R.; Edwards, C.A.; Combet, E.; Ramage, G. Utilising polyphenols for the clinical management of Candida albicans biofilms. Int. J. Antimicrob. Agents 2014, 44, 269–273. [Google Scholar] [CrossRef] [Green Version]
- Rane, H.S.; Bernardo, S.M.; Howell, A.B.; Lee, S.A. Cranberry-derived proanthocyanidins prevent formation of Candida albicans biofilms in artificial urine through biofilm- and adherence-specific mechanisms. J. Antimicrob. Chemother. 2014, 69, 428–436. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, A.; Khan, A.; Manzoor, N.; Khan, L.A. Evolution of ergosterol biosynthesis inhibitors as fungicidal against Candida. Microb. Pathog. 2010, 48, 35–41. [Google Scholar] [CrossRef]
- Mahata, D.; Mandal, S.M.; Bharti, R.; Gupta, V.K.; Mandal, M.; Nag, A.; Nando, G.B. Self-assembled cardanol azo derivatives as antifungal agent with chitin-binding ability. Int. J. Biol. Macromol. 2014, 69, 5–11. [Google Scholar] [CrossRef]
- Szczepaniak, J.; Łukaszewicz, M.; Krasowska, A. Detection of inhibitors of Candida albicans Cdr transporters using a diS-C3(3) fluorescence. Front. Microbiol. 2015, 6, 176. [Google Scholar] [CrossRef] [Green Version]
- Sharma, M.; Manoharlal, R.; Shukla, S.; Puri, N.; Prasad, T.; Ambudkar, S.V.; Prasad, R. Curcumin modulates efflux mediated by yeast ABC multidrug transporters and is synergistic with antifungals. Antimicrob. Agents Chemother. 2009, 53, 3256–3265. [Google Scholar] [CrossRef] [Green Version]
- Shapiro, H.M. Practical Flow Cytometry; Wiley: Hoboken, NJ, USA, 2003; ISBN 9780471411253. [Google Scholar]
- Dolab, J.; Lima, B.; Spaczynska, E.; Kos, J.; Cano, N.; Feresin, G.; Tapia, A.; Garibotto, F.; Petenatti, E.; Olivella, M.; et al. The Antimicrobial Activity of Annona emarginata (Schltdl.) H. Rainer and Most Active Isolated Compounds against Clinically Important Bacteria. Molecules 2018, 23, 1187. [Google Scholar] [CrossRef] [Green Version]
- Fonzi, W.; Irwin, M. Isogenic strain construction and gene mapping in Candida albicans. Genetics 1993, 134, 717–728. [Google Scholar]
- Sanglard, D.; Ischer, F. Susceptibilities of Candida albicans multidrug transporter mutants to various antifungal agents and other metabolic inhibitors. Antimicrob. Agents 1996, 40, 2300–2305. [Google Scholar] [CrossRef] [Green Version]
- Sanglard, D.; Ischer, F.; Monod, M.; Bille, J. Cloning of Candida albicans genes conferring resistance to azole antifungal agents: Characterization of CDR2, a new multidrug ABC transporter gene. Microbiology 1997, 143, 405–416. [Google Scholar] [CrossRef] [Green Version]
- Hall, M.J.; Middleton, R.F.; Westmacott, D. The fractional inhibitory concentration (FIC) index as a measure of synergy. J. Antimicrob. Chemother. 1983, 11, 427–433. [Google Scholar] [CrossRef]
- Hiller, D.; Sanglard, D.; Morschhäuser, J. Overexpression of the MDR1 gene is sufficient to confer increased resistance to toxic compounds in Candida albicans. Antimicrob. Agents Chemother. 2006, 50, 1365–1371. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, K.; Niimi, M.; Niimi, K.; Holmes, A.R.; Yates, J.E.; Decottignies, A.; Monk, B.C.; Goffeau, A.; Cannon, R.D. Functional expression of Candida albicans drug efflux pump Cdr1p in a Saccharomyces cerevisiae strain deficient in membrane transporters. Antimicrob. Agents Chemother. 2001, 45, 3366. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of the compounds are available from the authors. |






| Compound | WT (Wild Type Strain) | cdr1Δ | cdr2Δ | cdr1Δcdr2Δ |
|---|---|---|---|---|
| SQL1 | >138.8 | >138.8 | >138.8 | 138.8 |
| SQL2 | >122.12 | >122.12 | >122.12 | 122.12 |
| SQL 3 | 26.33 | 3.29 | 26.33 | 3.29 |
| SQL 4 | >122.12 | >122.12 | >122.12 | 122.12 |
| 8HQ | 17.42 | 36.29 | 17.42 | 17.42 |
| 8HQD | 19.10 | 39.79 | 19.10 | 19.10 |
| AmB | 0.9241 | 0.9241 | 0.9241 | 0.9241 |
| Fluconazole | 18.37 | 0.490 | 0.980 | 0.244 |
| Compound | WT (Wild Type Strain) | cdr1Δ | cdr2Δ | cdr1Δcdr2Δ |
|---|---|---|---|---|
| SQL 1 | 2 | 1 | 2 | 1 |
| SQL 2 | 2 | 0.5 | 2 | 0.5 |
| SQL 3 | 0.02 | 0.02 | 0.02 | 0.02 |
| SQL 4 | 1 | 0.5 | 0.5 | 0.5 |
| Strain | Genotype | Reference |
|---|---|---|
| CAF 2-1 | ura3Δ::imm434/URA3 | [48] |
| DSY 448 | cdr1Δ::hisG-URA3-hisG/cdr1Δ::hisG | [49] |
| DSY 653 | cdr2Δ::hisG-URA3-hisG/cdr2Δ::hisG | [50] |
| DSY 654 | cdr1Δ::hisG/cdr1Δ::hisG cdr2Δ::hisG-URA3-hisG/cdr2Δ::hisG | [50] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cieslik, W.; Szczepaniak, J.; Krasowska, A.; Musiol, R. Antifungal Styryloquinolines as Candida albicans Efflux Pump Inhibitors: Styryloquinolines are ABC Transporter Inhibitors. Molecules 2020, 25, 345. https://doi.org/10.3390/molecules25020345
Cieslik W, Szczepaniak J, Krasowska A, Musiol R. Antifungal Styryloquinolines as Candida albicans Efflux Pump Inhibitors: Styryloquinolines are ABC Transporter Inhibitors. Molecules. 2020; 25(2):345. https://doi.org/10.3390/molecules25020345
Chicago/Turabian StyleCieslik, Wioleta, Joanna Szczepaniak, Anna Krasowska, and Robert Musiol. 2020. "Antifungal Styryloquinolines as Candida albicans Efflux Pump Inhibitors: Styryloquinolines are ABC Transporter Inhibitors" Molecules 25, no. 2: 345. https://doi.org/10.3390/molecules25020345
APA StyleCieslik, W., Szczepaniak, J., Krasowska, A., & Musiol, R. (2020). Antifungal Styryloquinolines as Candida albicans Efflux Pump Inhibitors: Styryloquinolines are ABC Transporter Inhibitors. Molecules, 25(2), 345. https://doi.org/10.3390/molecules25020345