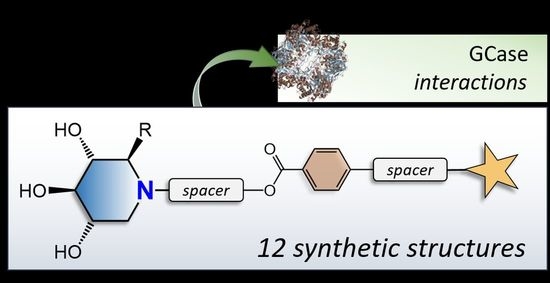
N-Alkylated Iminosugar Based Ligands: Synthesis and Inhibition of Human Lysosomal β-Glucocerebrosidase
Abstract
:1. Introduction
2. Results
2.1. Synthesis
2.2. Biological Evaluation
3. Materials and Methods
3.1. General Methods
3.2. General Synthetic Procedures
3.2.1. General Procedure A: (Mitsunobu Reaction)
3.2.2. General Procedure B: (Kornblum Oxidation)
3.2.3. General Procedure C: (Dess-Martin Oxidation)
3.2.4. General Procedure D: (Reductive Amination employing NaBH3CN)
3.3. Kinetic Studies
Specific Assay Conditions for Each Enzyme:
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Asano, N.; Nash, R.J.; Molyneux, R.J.; Fleet, G.W.J. Sugar-mimic glycosidase inhibitors: Natural occurrence, biological activity and prospects for therapeutic application. Tetrahedron: Asymmetry 2000, 11, 1645–1680. [Google Scholar] [CrossRef]
- Paulsen, H. Carbohydrates containing nitrogen or sulfur in the “hemiacetal” ring. Angew. Chem. Int. Ed. Engl. 1966, 5, 495–510. [Google Scholar] [CrossRef]
- Paulsen, H.; Sangster, I.; Heyns, K. Monosaccharide mit stickstoffhaltigem ring, XIII. Synthese und reaktionen von keto-piperidinosen. Chem. Ber. 1967, 100, 802–815. [Google Scholar] [CrossRef]
- Martin, O.R.; Compain, P. (Eds.) Iminosugars. From Synthesis to Therapeutic Applications; J. Wiley: Chichester, West Sussex, England; Hoboken, NJ, USA, 2007; ISBN 9780470033913. [Google Scholar]
- Stütz, A.E. (Ed.) Iminosugars as Glycosidase Inhibitors. Nojirimycin and Beyond, 1st ed.; Wiley-VCH: Weinheim, Germany, 1999; ISBN 3-527-29544-5. [Google Scholar]
- Stütz, A.E.; Wrodnigg, T.M. Carbohydrate-processing enzymes of the lysosome: Diseases caused by misfolded mutants and sugar mimetics as correcting pharmacological chaperones. Adv. Carbohydr. Chem. Biochem. 2016, 73, 225–302. [Google Scholar] [CrossRef]
- Nicolas, C.; Martin, O.R. Glycoside mimics from glycosylamines: Recent progress. Molecules 2018, 23, 1612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cipolla, L. (Ed.) Carbohydrate Chemistry: State of the Art and Challenges for Drug Development. An Overview on Structure, Biological Roles, Synthetic Methods and Application as Therapeutics; Imperial College Press: London, UK, 2016; ISBN 978-1-78326-719-4. [Google Scholar]
- Alonzi, D.S.; Scott, K.A.; Dwek, R.A.; Zitzmann, N. Iminosugar antivirals: The therapeutic sweet spot. Biochem. Soc. Trans. 2017, 45, 571–582. [Google Scholar] [CrossRef] [Green Version]
- Hossain, F.; Andreana, P.R. Developments in carbohydrate-based cancer therapeutics. Pharmaceuticals (Basel) 2019, 12, 84. [Google Scholar] [CrossRef] [Green Version]
- Wadood, A.; Ghufran, M.; Khan, A.; Azam, S.S.; Jelani, M.; Uddin, R. Selective glycosidase inhibitors: A patent review (2012-present). Int. J. Biol. Macromol. 2018, 111, 82–91. [Google Scholar] [CrossRef]
- Rauter, A.P.; Lindhorst, T. (Eds.) Carbohydrate Chemistry. Chemical and Biological Approaches: Vol. 38; Royal Society of Chemistry: Cambridge, UK, 2012; ISBN 978-1-84973-439-4. [Google Scholar]
- Willems, L.I.; Jiang, J.; Li, K.-Y.; Witte, M.D.; Kallemeijn, W.W.; Beenakker, T.J.N.; Schröder, S.P.; Aerts, J.M.F.G.; van der Marel, G.A.; Codée, J.D.C.; et al. From covalent glycosidase inhibitors to activity-based glycosidase probes. Chem. Eur. J. 2014, 20, 10864–10872. [Google Scholar] [CrossRef]
- Armstrong, Z.; Kuo, C.-L.; Lahav, D.; Liu, B.; Johnson, R.; Beenakker, T.J.M.; Boer, C.D.; Wong, C.-S.; van Rijssel, E.R.; Debets, M.F.; et al. Manno-epi-cyclophellitols enable activity-based protein profiling of human α-mannosidases and discovery of new golgi mannosidase II inhibitors. J. Am. Chem. Soc. 2020, 13021–13029. [Google Scholar] [CrossRef]
- Gueder, N.; Allan, G.; Telliez, M.-S.; Hague, F.; Fernandez, J.M.; Sanchez-Fernandez, E.M.; Ortiz-Mellet, C.; Ahidouch, A.; Ouadid-Ahidouch, H. sp (2)-Iminosugar α-glucosidase inhibitor 1-C-octyl-2-oxa-3-oxocastanospermine specifically affected breast cancer cell migration through stim1, β1-integrin, and FAK signaling pathways. J. Cell. Physiol. 2017, 232, 3631–3640. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-H.; Schelwies, M.; Enck, S.; Huang, L.-Y.; Huang, S.-H.; Chang, Y.-F.; Cheng, T.-J.R.; Cheng, W.-C.; Wong, C.-H. Iminosugar C-glycoside analogues of α-D-GlcNAc-1-phosphate: Synthesis and bacterial transglycosylase inhibition. J. Org. Chem. 2014, 79, 8629–8637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, L.; Yin, Z.; Niu, L.; Shao, J.; Chen, H.; Li, X. Synthesis of pentacyclic iminosugars with constrained butterfly-like conformation and their HIV-RT inhibitory activity. Bioorg. Med. Chem. Lett. 2018, 28, 425–428. [Google Scholar] [CrossRef] [PubMed]
- Wetherill, L.F.; Wasson, C.W.; Swinscoe, G.; Kealy, D.; Foster, R.; Griffin, S.; Macdonald, A. Alkyl-imino sugars inhibit the pro-oncogenic ion channel function of human papillomavirus (HPV) E5. Antiviral Res. 2018, 158, 113–121. [Google Scholar] [CrossRef]
- Tyrrell, B.E.; Sayce, A.C.; Warfield, K.L.; Miller, J.L.; Zitzmann, N. Iminosugars: Promising therapeutics for influenza infection. Crit. Rev. Microbiol. 2017, 43, 521–545. [Google Scholar] [CrossRef] [Green Version]
- Jacob, J.R.; Mansfield, K.; You, J.E.; Tennant, B.C.; Kim, Y.H. Natural iminosugar derivatives of 1-deoxynojirimycin inhibit glycosylation of hepatitis viral envelope proteins. J. Microbiol. 2007, 45, 431–440. [Google Scholar]
- Miller, J.L.; Tyrrell, B.E.; Zitzmann, N. Mechanisms of Antiviral Activity of Iminosugars Against Dengue Virus. Adv. Exp. Med. Biol. 2018, 1062, 277–301. [Google Scholar] [CrossRef]
- Evans, G.B.; Tyler, P.C.; Schramm, V.L. Immucillins in infectious diseases. ACS Infect. Dis. 2018, 4, 107–117. [Google Scholar] [CrossRef]
- Chavan, S.R.; Gavale, K.S.; Khan, A.; Joshi, R.; Kumbhar, N.; Chakravarty, D.; Dhavale, D.D. Iminosugars spiro-linked with morpholine-fused 1,2,3-triazole: Synthesis, conformational analysis, glycosidase inhibitory activity, antifungal assay, and docking studies. ACS Omega 2017, 2, 7203–7218. [Google Scholar] [CrossRef]
- Sánchez-Fernández, E.M.; García Fernández, J.M.; Mellet, C.O. Glycomimetic-based pharmacological chaperones for lysosomal storage disorders: Lessons from gaucher, GM1-gangliosidosis and fabry diseases. Chem. Commun. (Camb) 2016, 52, 5497–5515. [Google Scholar] [CrossRef] [Green Version]
- Harit, V.K.; Ramesh, N.G. Amino-functionalized iminocyclitols: Synthetic glycomimetics of medicinal interest. RSC Adv. 2016, 6, 109528–109607. [Google Scholar] [CrossRef]
- Wennekes, T.; van den Berg, R.J.B.H.N.; Boot, R.G.; van der Marel, G.A.; Overkleeft, H.S.; Aerts, J.M.F.G. Glycosphingolipids—nature, function, and pharmacological modulation. Angew. Chem. Int. Ed. Engl. 2009, 48, 8848–8869. [Google Scholar] [CrossRef] [PubMed]
- Convertino, M.; Das, J.; Dokholyan, N.V. Pharmacological chaperones: Design and development of new therapeutic strategies for the treatment of conformational diseases. ACS Chem. Biol. 2016, 11, 1471–1489. [Google Scholar] [CrossRef] [PubMed]
- CAZy-Home. Available online: http://www.cazy.org/ (accessed on 27 July 2020).
- Katakam, K. Overview of gaucher disease and its management. WJPR 2017, 563–584. [Google Scholar] [CrossRef] [Green Version]
- Aziz, O.; Bürli, R.W.; Fischer, D.F.; Frearson, J.; Wall, M.D. Towards Small Molecules as Therapies for Alzheimer’s Disease and Other Neurodegenerative Disorders. In Drug Design and Discovery in Alzheimer’s Disease; Atta-ur-Rahman, Choudhary, M.I., Eds.; Elsevier Science: Burlington, NJ, USA, 2015; pp. 199–290. ISBN 9780128039595. [Google Scholar]
- Gatto, E.M.; Da Prat, G.; Etcheverry, J.L.; Drelichman, G.; Cesarini, M. Parkinsonisms and glucocerebrosidase deficiency: A comprehensive review for molecular and cellular mechanism of glucocerebrosidase deficiency. Brain Sci. 2019, 9, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wrodnigg, T.M.; Stütz, A.E. The Two Faces of Iminoalditols: Powerful inhibitors trigger glycoside activation. Curr. Enzym. Inhib. 2012, 8, 47–99. [Google Scholar] [CrossRef]
- Parmeggiani, C.; Catarzi, S.; Matassini, C.; D’Adamio, G.; Morrone, A.; Goti, A.; Paoli, P.; Cardona, F. Human Acid β-Glucosidase inhibition by carbohydrate derived iminosugars: Towards new pharmacological chaperones for gaucher disease. ChemBioChem 2015, 16, 2054–2064. [Google Scholar] [CrossRef]
- Tamburrini, A.; Colombo, C.; Bernardi, A. Design and synthesis of glycomimetics: Recent advances. Med. Res. Rev. 2020, 40, 495–531. [Google Scholar] [CrossRef]
- Oulaïdi, F.; Front-Deschamps, S.; Gallienne, E.; Lesellier, E.; Ikeda, K.; Asano, N.; Compain, P.; Martin, O.R. Second-generation iminoxylitol-based pharmacological chaperones for the treatment of Gaucher disease. ChemMedChem 2011, 6, 353–361. [Google Scholar] [CrossRef]
- Zoidl, M.; Wolfsgruber, A.; Schalli, M.; Nasseri, S.A.; Weber, P.; Stütz, A.E.; Withers, S.G.; Wrodnigg, T.M. Synthesis of modified 1,5-imino-D-xylitols as ligands for lysosomal β-glucocerebrosidase. Monatsh. Chem. 2019, 150, 831–842. [Google Scholar] [CrossRef] [Green Version]
- Fröhlich, R.F.G.; Furneaux, R.H.; Mahuran, D.J.; Rigat, B.A.; Stütz, A.E.; Tropak, M.B.; Wicki, J.; Withers, S.G.; Wrodnigg, T.M. 1-Deoxynojirimycins with dansyl capped N-substituents as probes for Morbus Gaucher affected cell lines. Carbohydr. Res. 2010, 345, 1371–1376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mena-Barragán, T.; García-Moreno, M.I.; Sevšek, A.; Okazaki, T.; Nanba, E.; Higaki, K.; Martin, N.I.; Pieters, R.J.; Fernández, J.M.G.; Mellet, C.O. Probing the Inhibitor versus Chaperone Properties of sp2-Iminosugars towards Human β-Glucocerebrosidase: A picomolar chaperone for gaucher disease. Molecules 2018, 23, 927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schönemann, W.; Gallienne, E.; Ikeda-Obatake, K.; Asano, N.; Nakagawa, S.; Kato, A.; Adachi, I.; Górecki, M.; Frelek, J.; Martin, O.R. Glucosylceramide mimics: Highly potent GCase inhibitors and selective pharmacological chaperones for mutations associated with types 1 and 2 Gaucher disease. ChemMedChem 2013, 8, 1805–1817. [Google Scholar] [CrossRef] [PubMed]
- Serra-Vinardell, J.; Díaz, L.; Casas, J.; Grinberg, D.; Vilageliu, L.; Michelakakis, H.; Mavridou, I.; Aerts, J.M.F.G.; Decroocq, C.; Compain, P.; et al. Glucocerebrosidase enhancers for selected Gaucher disease genotypes by modification of α-1-C-substituted imino-d-xylitols (DIXs) by click chemistry. ChemMedChem 2014, 9, 1744–1754. [Google Scholar] [CrossRef]
- Compain, P.; Martin, O.R.; Boucheron, C.; Godin, G.; Yu, L.; Ikeda, K.; Asano, N. Design and synthesis of highly potent and selective pharmacological chaperones for the treatment of Gaucher’s disease. ChemBioChem 2006, 7, 1356–1359. [Google Scholar] [CrossRef]
- Ghisaidoobe, A.; Bikker, P.; de Bruijn, A.C.J.; Godschalk, F.D.; Rogaar, E.; Guijt, M.C.; Hagens, P.; Halma, J.M.; van’t Hart, S.M.; Luitjens, S.B.; et al. Identification of potent and selective glucosylceramide synthase inhibitors from a library of N-alkylated iminosugars. ACS Med. Chem. Lett. 2011, 2, 119–123. [Google Scholar] [CrossRef] [Green Version]
- Ghisaidoobe, A.T.; van den Berg, R.J.B.H.N.; Butt, S.S.; Strijland, A.; Donker-Koopman, W.E.; Scheij, S.; van den Nieuwendijk, A.M.C.H.; Koomen, G.-J.; van Loevezijn, A.; Leemhuis, M.; et al. Identification and development of biphenyl substituted iminosugars as improved dual glucosylceramide synthase/neutral glucosylceramidase inhibitors. J. Med. Chem. 2014, 57, 9096–9104. [Google Scholar] [CrossRef]
- Wennekes, T.; van den Berg, R.J.B.H.N.; Donker, W.; van der Marel, G.A.; Strijland, A.; Aerts, J.M.F.G.; Overkleeft, H.S. Development of adamantan-1-yl-methoxy-functionalized 1-deoxynojirimycin derivatives as selective inhibitors of glucosylceramide metabolism in man. J. Org. Chem. 2007, 72, 1088–1097. [Google Scholar] [CrossRef]
- Hoogendoorn, S.; Mock, E.D.; Strijland, A.; Donker-Koopman, W.E.; van den Elst, H.; van den Berg, R.J.B.H.N.; Aerts, J.M.F.G.; van der Marel, G.A.; Overkleeft, H.S. ortho-Carborane-Modified N-Substituted Deoxynojirimycins. Eur. J. Org. Chem. 2015, 2015, 4437–4446. [Google Scholar] [CrossRef]
- Castellan, T.; Santos, C.; Rodriguez, F.; Lepage, M.L.; Liang, Y.; Bodlenner, A.; Compain, P.; Génisson, Y.; Dehoux, C.; Ballereau, S. N,O-Dialkyl deoxynojirimycin derivatives as CERT START domain ligands. Bioorg. Med. Chem. Lett. 2020, 30, 126796. [Google Scholar] [CrossRef]
- González-Cuesta, M.; Ortiz Mellet, C.; García Fernández, J.M. Carbohydrate supramolecular chemistry: Beyond the multivalent effect. Chem. Commun. (Camb) 2020, 56, 5207–5222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Compain, P. Multivalent Effect in glycosidase inhibition: The end of the beginning. Chem. Rec. 2020, 20, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Zugenmaier, P.; Bock, I.; Schacht, J. The molecular and crystal structures of three 4-(ω-Cyanoalkyloxy)benzoic acids and of A 1:1 ordered mixed crystal of 4-(ω-Cyanobutyloxy)benzoic acid and 4-(ω-Cyanopentyloxy)benzoic acid formed by hydrogen bonding. Mol. Cryst. Liq. Cryst. 2003, 392, 31–47. [Google Scholar] [CrossRef]
- Kornblum, N.; Powers, J.W.; Anderson, G.J.; Jones, W.J.; Larson, H.O.; Levand, O.; Weaver, W.M. A new and selective method of oxidation. J. Am. Chem. Soc. 1957, 79, 6562. [Google Scholar] [CrossRef]
- Kornblum, N.; Jones, W.J.; Anderson, G.J. A New and selective method of oxidation. the conversion of alkyl halides and alkyl tosylates to aldehydes. J. Am. Chem. Soc. 1959, 81, 4113–4114. [Google Scholar] [CrossRef]
- Demko, Z.P.; Sharpless, K.B. A click chemistry approach to tetrazoles by huisgen 1,3-dipolar cycloaddition: Synthesis of 5-acyltetrazoles from azides and acyl cyanides. Angew. Chem. Int. Ed. 2002, 41, 2113–2116. [Google Scholar] [CrossRef]
- Aldhoun, M.; Massi, A.; Dondoni, A. Click azide-nitrile cycloaddition as a new ligation tool for the synthesis of tetrazole-tethered C-glycosyl alpha-amino acids. J. Org. Chem. 2008, 73, 9565–9575. [Google Scholar] [CrossRef]
- Della Volpe, S.; Nasti, R.; Queirolo, M.; Unver, M.Y.; Jumde, V.K.; Dömling, A.; Vasile, F.; Potenza, D.; Ambrosio, F.A.; Costa, G.; et al. Novel compounds targeting the RNA-binding protein HuR. structure-based design, synthesis, and interaction studies. ACS Med. Chem. Lett. 2019, 10, 615–620. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.; Zhan, C.; You, Y.; Zhang, H.; Ma, J.; Xiong, Z.; Liu, X.; Wei, R. Synthesis and photoinduced anisotropy of polymers containing nunchaku-like Unit with an azobenzene and a mesogen. Polymers (Basel) 2019, 11, 600. [Google Scholar] [CrossRef] [Green Version]
- Lebeau, L.; Oudet, P.; Mioskowski, C. Synthesis of new phospholipids linked to steroid-hormone derivatives designed for two-dimensional crystallization of proteins. Helv. Chim. Acta 1991, 74, 1697–1706. [Google Scholar] [CrossRef]
- Breugst, M.; Reissig, H.-U. The huisgen reaction: Milestones of the 1,3-dipolar cycloaddition. Angew. Chem. Int. Ed. Engl. 2020. [Google Scholar] [CrossRef] [Green Version]
- Schitter, G.; Steiner, A.J.; Pototschnig, G.; Scheucher, E.; Thonhofer, M.; Tarling, C.A.; Withers, S.G.; Fantur, K.; Paschke, E.; Mahuran, D.J.; et al. Fluorous iminoalditols: A new family of glycosidase inhibitors and pharmacological chaperones. ChemBioChem 2010, 11, 2026–2033. [Google Scholar] [CrossRef] [Green Version]
- Tu, Z.; Li, S.; Cui, J.; Xu, J.; Taylor, M.; Ho, D.; Luedtke, R.R.; Mach, R.H. Synthesis and pharmacological evaluation of fluorine-containing D3 dopamine receptor ligands. J. Med. Chem. 2011, 54, 1555–1564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stamford, A.; Miller, M.W.; Demong, D.E.; Greenlee, W.J.; Kozlowski, J.A.; Lavey, B.J.; Wong, M.K.C.; Yu, W.; Dai, X.; Yang, D.Y.; et al. Spiro-Imidazolone Derivatives as Glucagon Receptor Antagonists. U.S. Patent WO2010039789A1, 30 September 2009. [Google Scholar]
- Dax, K.; Gaigg, B.; Grassberger, V.; Kölblinger, B.; Stütz, A.E. Einfache synthesen von 1,5-didesoxy-1,5-imino-d-glucit (1-desoxynojirimycin) und 1,6-didesoxy-1,6-imino-d-glucit aus d-glucofuranurono-6,3-Lacton. J. Carbohydr. Chem. 1990, 9, 479–499. [Google Scholar] [CrossRef]
- Häusler, H.; Rupitz, K.; Stütz, A.E.; Withers, S.G. N-alkylated derivatives of 1,5-dideoxy-1,5-iminoxylitol as β-xylosidase and β-glucosidase inhibitors. Monatsh. Chem. 2002, 133, 555–560. [Google Scholar] [CrossRef]
- Greimel, P.; Häusler, H.; Lundt, I.; Rupitz, K.; Stütz, A.E.; Tarling, C.A.; Withers, S.G.; Wrodnigg, T.M. Fluorescent glycosidase inhibiting 1,5-dideoxy-1,5-iminoalditols. Bioorg. Med. Chem. Lett. 2006, 16, 2067–2070. [Google Scholar] [CrossRef]
- Prade, H.; Mackenzie, L.F.; Withers, S.G. Enzymatic synthesis of disaccharides using agrobacterium sp. β-glucosidase. Carbohydr. Res. 1997, 305, 371–381. [Google Scholar] [CrossRef]
- Kempton, J.B.; Withers, S.G. Mechanism of Agrobacterium beta-glucosidase: Kinetic studies. Biochemistry 1992, 31, 9961–9969. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |











| Compound | Enzyme (GH Family) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
 | ||||||||||
| β-Glc/Gal | β-Gal | β-Gal | α-Gal | α-Glc | β-Glc | |||||
| R = | Config. | Nr. | Abg (GH1) | E. coli (GH2) | Bovine liv. (GH35) | Fabrazyme (GH27) | S. cer. (GH13) | GCase (GH30) | ||
 | Glc | 45 | 1.31 | N.I. | 2.42 | N.I. | 23.7 | 0.28 | ||
| Xyl | 46 | 12.10 | N.I. | 7.76 | N.I. | N.I. | 0.62 | |||
 | Glc | 47 | 1.70 | N.I. | 1.74 | N.I. | 221 | 0.09 | ||
| Xyl | 48 | 32.6 | N.I. | 11.76 | N.I. | N.I. | 1.40 | |||
 | Glc | 49 | 3.60 | N.I. | 6.94 | N.I. | N.I. | 0.35 | ||
| Xyl | 50 | 23.70 | 255 | 8.36 | N.I. | N.I. | 0.40 | |||
 | Glc | 51 | 0.0022 | N.I. | 3.25 | N.I. | 4.9 | 0.37 | ||
| Xyl | 52 | 17 | N.I. | 7.41 | N.I. | 267 | 0.74 | |||
 | Glc | 53 | 0.06 | 424 | 2.13 | N.I. | 8.1 | 0.022 | ||
| Xyl | 54 | 0.58 | N.I. | 4.65 | N.I. | N.I. | 0.31 | |||
 | Glc | 55 | 0.06 | 89 | 1.82 | N.I. | 8.7 | 0.018 | ||
| Xyl | 56 | 0.76 | 198 | 10.36 | N.I. | N.I. | 2.60 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wolfsgruber, A.; Thonhofer, M.; Weber, P.; Nasseri, S.A.; Fischer, R.; Schalli, M.; Stütz, A.E.; Withers, S.G.; Wrodnigg, T.M. N-Alkylated Iminosugar Based Ligands: Synthesis and Inhibition of Human Lysosomal β-Glucocerebrosidase. Molecules 2020, 25, 4618. https://doi.org/10.3390/molecules25204618
Wolfsgruber A, Thonhofer M, Weber P, Nasseri SA, Fischer R, Schalli M, Stütz AE, Withers SG, Wrodnigg TM. N-Alkylated Iminosugar Based Ligands: Synthesis and Inhibition of Human Lysosomal β-Glucocerebrosidase. Molecules. 2020; 25(20):4618. https://doi.org/10.3390/molecules25204618
Chicago/Turabian StyleWolfsgruber, Andreas, Martin Thonhofer, Patrick Weber, Seyed A. Nasseri, Roland Fischer, Michael Schalli, Arnold E. Stütz, Stephen G. Withers, and Tanja M. Wrodnigg. 2020. "N-Alkylated Iminosugar Based Ligands: Synthesis and Inhibition of Human Lysosomal β-Glucocerebrosidase" Molecules 25, no. 20: 4618. https://doi.org/10.3390/molecules25204618
APA StyleWolfsgruber, A., Thonhofer, M., Weber, P., Nasseri, S. A., Fischer, R., Schalli, M., Stütz, A. E., Withers, S. G., & Wrodnigg, T. M. (2020). N-Alkylated Iminosugar Based Ligands: Synthesis and Inhibition of Human Lysosomal β-Glucocerebrosidase. Molecules, 25(20), 4618. https://doi.org/10.3390/molecules25204618