Characterization of Modified Natural Minerals and Rocks for Possible Adsorption and Catalytic Use
Abstract
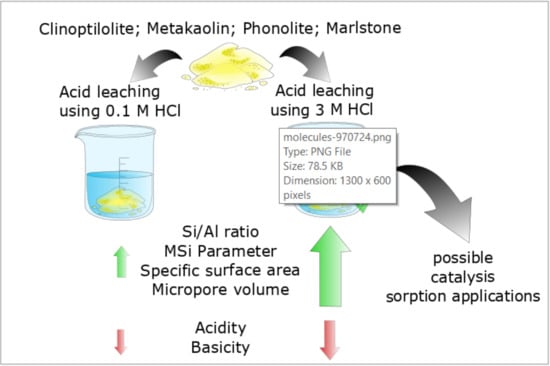
:1. Introduction
2. Results and Discussion
2.1. Characterization of Materials
2.2. Adsorption Tests
3. Materials and Methods
3.1. Materials
3.2. Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Polat, E.; Karaca, M.; Demir, H.; Onus, A.N. Use of natural zeolite (clinoptilolite) in agriculture. J. Fruit Ornam. Plant Res. 2004, 12, 183–189. [Google Scholar]
- Ahmadi, B.; Shekarchi, M. Use of natural zeolite as a supplementary cementitious material. Cem. Concr. Compos. 2010, 32, 134–141. [Google Scholar] [CrossRef]
- Kraljević Pavelić, A.; Simović Medica, J.; Gumbarević, D.; Filošević, A.; Pržulj, N.; Pavelić, K. Critical Review on Zeolite Clinoptilolite Safety and Medical Applications in vivo. Front. Pharmacol. 2018, 9, 1350. [Google Scholar] [CrossRef] [PubMed]
- Roque-Malherbe, R. Applications of Natural Zeolites in Pollution Abatement and Industry. Handb. Surf. Interfaces Mater. 2001, 5, 495–522. [Google Scholar] [CrossRef]
- Inglezakis, V.J.; Loizidou, M.D.; Grigoropoulou, H.P. Ion exchange of Pb2+, Cu2+, Fe3+, and Cr3+ on natural clinoptilolite: Selectivity determination and influence of acidity on metal uptake. J. Colloid Interface Sci. 2003, 261, 49–54. [Google Scholar] [CrossRef]
- Stylianou, M.A.; Hadjiconstantinou, M.P.; Inglezakis, V.J.; Moustakas, K.G.; Loizidou, M.D. Use of natural clinoptilolite for the removal of lead, copper and zinc in fixed bed column. J. Hazard. Mater. 2007, 143, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Domaracka, L.; Strba, L.; Krsak, B.; Muchova, M. Possibilities of profitable business with nature zeolite—An example from the Nižný Hrabovec Deposit, Slovakia. Int. J. Sci. Res. Innov. Tech. 2015, 2, 12–19. [Google Scholar]
- Wibowo, E.; Rokhmat, M.; Abdullah, M. Reduction of seawater salinity by natural zeolite (Clinoptilolite): Adsorption isotherms, thermodynamics and kinetics. Desalination 2017, 409, 146–156. [Google Scholar] [CrossRef]
- Doula, M.K. Simultaneous removal of Cu, Mn and Zn from drinking water with the use of clinoptilolite and its Fe-modified form. Water Res. 2009, 43, 3659–3672. [Google Scholar] [CrossRef]
- Burris, L.E.; Juenger, M.C.G. Effect of calcination on the reactivity of natural clinoptilolite zeolites used as supplementary cementitious materials. Constr. Build. Mater. 2020, 258, 11998. [Google Scholar] [CrossRef]
- Qin, Z.; Ma, C.; Zheng, Z. Effects of metakaolin on properties and microstructure of magnesium phosphate cement. Constr. Build. Mater. 2020, 234, 117353. [Google Scholar] [CrossRef]
- Potapova, E.; Dmitrieva, E. The Metakaolin—A New Hydraulically Active Pozzolanic Additive. Mater. Sci. Forum 2019, 974, 319–324. [Google Scholar] [CrossRef]
- Liew, Y.M.; Heah, C.Y.; Mohd Mustafa, A.B.; Kamarudin, H. Structure and properties of clay-based geopolymer cements: A review. Prog. Mater. Sci. 2016, 83, 595–629. [Google Scholar] [CrossRef]
- Sabir, B.B.; Wild, S.; Bai, J. Metakaolin and calcined clays as pozzolans for concrete: A review. Cem. Concr. Comp. 2001, 23, 441–454. [Google Scholar] [CrossRef]
- Přikryl, R.; Přikrylová, J.; Racek, M.; Weishauptová, Z.; Kreislová, K. Decay mechanism of indoor porous opuka stone: A case study from the main altar located in the St. Vitus Cathedral, Prague (Czech Republic). Environ. Earth Sci. 2017, 76, 290. [Google Scholar] [CrossRef]
- Přikryl, R.; Racek, M.; Přikrylová, J.; Weishauptová, Z. Understanding nature and properties of “opuka” stone, extraordinary natural stone of medieval Central Europe. In 20th EGU General Assembly, Proceedings of the Conference Held, Vienna, Austria, 4–13 April 2018; EGU: Munich, Germany, 2018; p. 18889. [Google Scholar]
- Hidalgo-Herrador, J.M.; Tišler, Z.; Hajková, P.; Soukupová, L.; Zárybnická, L.; Černá, K. Cold Plasma and Acid Treatment Modification Effects on Phonolite. Acta Chim. Slov. 2017, 64, 598–602. [Google Scholar] [CrossRef] [Green Version]
- Tišler, Z.; Šafář, J.; Velvarská, R.; Pelíšková, L. Modifikované alkalicky aktivované zeolitové pěny: Příprava a charakterizace. Chem. Listy 2019, 113, 111–116. [Google Scholar]
- Wahono, S.K.; Prasetyo, D.J.; Jatmiko, T.H.; Pratiwi, D.; Suwanto, A.; Hernawan; Vasilev, K. Multi-stage. dealumination for characteristic engineering of mordenite-clinoptilolite natural zeolite. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2019; Volume 2085, p. 020044. [Google Scholar] [CrossRef]
- Hrachovcová, K.; Tišler, Z.; Svobodová, E.; Šafář, J. Modified Alkali Activated Zeolite Foams with Improved Textural and Mechanical Properties. Minerals 2020, 10, 483. [Google Scholar] [CrossRef]
- Beyer, H.K. Dealumination Techniques for Zeolites. Mol. Sieves 2002, 3, 204–254. [Google Scholar] [CrossRef]
- Yu, C.H.; Huang, C.H.; Tan, C.S. A Review of CO2 Capture by Absorption and Adsorption. Aerosol. Air. Qual. Res. 2012, 12, 745–769. [Google Scholar] [CrossRef] [Green Version]
- Bonenfant, D.; Kharoune, M.; Niquette, P.; Mimeault, M.; Hausler, R. Advances in principal factors influencing carbon dioxide adsorption on zeolites. Sci. Technol. Adv. Mater. 2008, 9, 013007. [Google Scholar] [CrossRef]
- Younas, M.; Sohail, M.; Leong, L.K.; Bashir, M.J.; Sumathi, S. Feasibility of CO2 adsorption by solid adsorbents: A review on low-temperature systems. Int. J. Environ. Sci. Technol. 2016, 13, 1839–1860. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Basabe, Y.; Rodriguez-Iznaga, I.; de Menorval, L.C.; Llewellyn, P.; Maurin, G.; Lewis, D.W.; Binions, R.; Autie, M.; Ruiz-Salvador, A.R. Step-wise dealumination of natural clinoptilolite: Structural and physicochemical characterization. Microporous Mesoporous Mater. 2010, 135, 187–196. [Google Scholar] [CrossRef]
- Tschegg, C.; Rice, A.H.N.; Grasemann, B.; Matiasek, E.; Kobulej, P.; Dzivák, M.; Berger, T. Petrogenesis of a Large-Scale Miocene Zeolite Tuff in the Eastern Slovak Republic: The Nižný Hrabovec Open-Pit Clinoptilolite Mine. Econ. Geol. 2019, 114, 1177–1194. [Google Scholar] [CrossRef] [Green Version]
- Salahudeen, N.; Ahmed, A.S.; Al-Muhtaseb, A.H.; Dauda, M.; Waziri, S.M.; Jibril, B.Y. Synthesis and Characterization of Micro-Sized Silica from Kankara Kaolin. J. Eng. Res. 2014, 19, 27–32. [Google Scholar]
- Yaseri, Y.; Hajiaghaei, G.; Mohammadi, F.; Mahdikhani, M.; Farokhzad, R. The role of synthesis parameters on the workability, setting and strength properties of binary binder based geopolymer paste. Constr. Build. Mater. 2017, 157, 534–545. [Google Scholar] [CrossRef]
- Alothman, Z.A. A Review: Fundamental Aspects of Silicate Mesoporous Materials. Materials 2012, 5, 2874–2902. [Google Scholar] [CrossRef] [Green Version]
- Tang, X.; Jiang, Z.; Li, Z.; Gao, Z.; Bai, Y.; Zhao, S.; Feng, J. The effect of the variation in material composition on the heterogeneous pore structure of high-maturity shale of the Silurian Longmaxi formation in the southeastern Sichuan Basin, China. J. Nat. Gas Sci. Eng. 2015, 23, 464–473. [Google Scholar] [CrossRef]
- Tišler, Z.; Horáček, J.; Šafář, J.; Velvarska, R.; Peliskova, L.; Kocik, J.; Gherib, Y.; Marklova, K.; Bulanek, R.; Kubicka, D. Clinoptilolite foams prepared by alkali activation of natural zeolite and their post synthesis modifications. Microporous Mesoporous Mater. 2019, 282, 169–179. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, M.; Yu, Y. Distribution of aluminum and its influence on the acid strength of Y zeolite. Microporous Mesoporous Mater. 2013, 169, 47–53. [Google Scholar] [CrossRef]
- Gao, Y.; Zheng, B.; Wu, G.; Ma, F.; Liu, C. Effect of the Si/Al ratio on the performance of hierarchical ZSM-5 zeolites for methanol aromatization. RSC Adv. 2016, 6, 83581–83588. [Google Scholar] [CrossRef]
- Krivácsy, Z.; Hlavay, J. A simple method for the determination of clinoptilolite in natural zeolite rocks. Zeolites 1995, 15, 551–555. [Google Scholar] [CrossRef]
- Cakicioglu-Ozkan, F.; Ulku, S. The effect of HCl treatment on water vapor adsorption characteristics of clinoptilolite rich natural zeolite. Microporous Mesoporous Mater. 2005, 77, 47–53. [Google Scholar] [CrossRef] [Green Version]
- Tišler, Z.; Hrachovcová, K.; Svobodová, E.; Šafář, J.; Pelíšková, L. Acid and Thermal Treatment of Alkali-Activated Zeolite Foams. Minerals 2019, 9, 719. [Google Scholar] [CrossRef] [Green Version]
- Belmokhtar, N.; Ammari, M.; Brigui, J.; Ben allal, L. Comparison of the microstructure and the compressive strength of two geopolymers derived from Metakaolin and an industrial sludge. Constr. Build. Mater. 2017, 146, 621–629. [Google Scholar] [CrossRef]
- Lancellotti, I.; Catauro, M.; Ponzoni, C.; Bollino, F.; Leonelli, C. Inorganic polymers from alkali activation of metakaolin: Effect of setting and curing on structure. J. Solid State Chem. 2013, 200, 341–348. [Google Scholar] [CrossRef]
- Dumitriu, E.; Cobzaru, C.; Hulea, V.; Oprea, S. Dealuminated Natural Zeolites for Applications in Catalytic Processes with Formation of C-C Bonds. Rev. Chim. 2010, 61, 400–403. [Google Scholar]
- Kusuma, R.I.; Hadinoto, J.P.; Ayucitra, A.; Soetaredjo, F.E.; Ismadji, S. Natural zeolite from Pacitan Indonesia, as catalyst support for transesterification of palm oil. Appl. Clay Sci. 2013, 74, 121–126. [Google Scholar] [CrossRef] [Green Version]
- Arcoya, A.; Gonzalez, J.A.; Travieso, N.; Seoane, X.L. Physicochemical and catalytic properties of a modified natural clinoptilolite. Clay Miner. 1994, 29, 123–131. [Google Scholar] [CrossRef]
- Hidalgo, J.M.; Tišler, Z.; Vráblík, A.; Velvarská, R.; Lederer, J. Acid-modified phonolite and foamed zeolite as supports for NiW catalysts for deoxygenation of waste rendering fat. React. Kinet. Mech. Catal. 2019, 126, 773–793. [Google Scholar] [CrossRef]







| Sample | Specific Surface Area-SBET (m2/g) | Surface Area (m2/g) * | Total Pore Volume (cm3/g) * | Micropore Volume (cm3/g) * | Mesopore Volume (cm3/g) * |
|---|---|---|---|---|---|
| CLI/S | 24.6 | 28.7 | 0.082 | 0.005 | 0.055 |
| CLI/D1 | 82.1 | 114.3 | 0.163 | 0.007 | 0.120 |
| CLI/D2 | 186.3 | 297.7 | 0.206 | 0.051 | 0.123 |
| MK/S | 13.1 | 10.8 | 0.042 | 0.000 | 0.033 |
| MK/D1 | 23.8 | 23.4 | 0.049 | 0.003 | 0.036 |
| MK/D2 | 226.5 | 300.5 | 0.153 | 0.067 | 0.070 |
| PH/S | 7.6 | 4.9 | 0.011 | 0.000 | 0.009 |
| PH/D1 | 13.1 | 9.2 | 0.017 | 0.000 | 0.015 |
| PH/D2 | 120.0 | 163.9 | 0.096 | 0.024 | 0.067 |
| MRL/S | 28.2 | 26.2 | 0.137 | 0.000 | 0.096 |
| MRL/D1 | 33.6 | 31.2 | 0.158 | 0.000 | 0.113 |
| MRL/D2 | 31.2 | 30.1 | 0.170 | 0.000 | 0.120 |
| Sample | Csum (mmol/g) | Tmax1 (°C) | Tmax2 (°C) |
|---|---|---|---|
| CLI/S | 1.252 | 172 | 309 |
| CLI/D1 | 1.100 | 188 | 337 |
| CLI/D2 | 0.290 | 153 | 158 |
| MK/S | 0.053 | 150 | - |
| MK/D1 | 0.141 | 147 | - |
| MK/D2 | 0.112 | 138 | - |
| PH | 0.043 | 228 | - |
| PH/D1 | 0.045 | 229 | - |
| PH/D2 | 0.008 | 128 | - |
| MRL | 0.058 | 169 | - |
| MRL/D1 | 0.056 | 172 | - |
| MRL/D2 | 0.035 | 166 | - |
| Sample | CO2 Adsorbed (wt%) |
|---|---|
| CLI/S | 1.8 |
| CLI/D1 | 2.1 |
| CLI/D2 | 1.1 |
| MK/S | 0.4 |
| MK/D1 | 0.2 |
| MK/D2 | 0.7 |
| PH/S | 0.1 |
| PH/D1 | 0.1 |
| PH/D2 | 0.6 |
| MRL/S | 0.2 |
| MRL/D1 | 0.1 |
| MRL/D2 | 0.1 |
Sample Availability: Samples of the compounds CLI/S, MK/S, PH/S and MRL/S are available from the authors. Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strejcová, K.; Tišler, Z.; Svobodová, E.; Velvarská, R. Characterization of Modified Natural Minerals and Rocks for Possible Adsorption and Catalytic Use. Molecules 2020, 25, 4989. https://doi.org/10.3390/molecules25214989
Strejcová K, Tišler Z, Svobodová E, Velvarská R. Characterization of Modified Natural Minerals and Rocks for Possible Adsorption and Catalytic Use. Molecules. 2020; 25(21):4989. https://doi.org/10.3390/molecules25214989
Chicago/Turabian StyleStrejcová, Kateřina, Zdeněk Tišler, Eliška Svobodová, and Romana Velvarská. 2020. "Characterization of Modified Natural Minerals and Rocks for Possible Adsorption and Catalytic Use" Molecules 25, no. 21: 4989. https://doi.org/10.3390/molecules25214989
APA StyleStrejcová, K., Tišler, Z., Svobodová, E., & Velvarská, R. (2020). Characterization of Modified Natural Minerals and Rocks for Possible Adsorption and Catalytic Use. Molecules, 25(21), 4989. https://doi.org/10.3390/molecules25214989