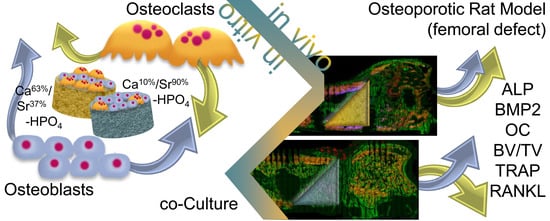
Gelatin-Modified Calcium/Strontium Hydrogen Phosphates Stimulate Bone Regeneration in Osteoblast/Osteoclast Co-Culture and in Osteoporotic Rat Femur Defects—In Vitro to In Vivo Translation
Abstract
:1. Introduction
2. Results
2.1. In Vitro Analysis of Material Properties and Cellular Reaction
2.1.1. Morphological Characterization of Calcium/Strontium Hydrogen Phosphates and Specimen
2.1.2. Mechanical Strength and Ion Release of Calcium/Strontium Hydrogen Phosphates
2.1.3. Osteoblast/Osteoclast Co-Culture
2.2. Animal Experiments
2.2.1. General Information
2.2.2. µ-CT Analysis
2.2.3. ToF-SIMS
2.2.4. Histomorphometry and Histology
2.2.5. Immunohistochemical Analysis
2.2.6. Molecular Biology
3. Discussion
4. Materials and Methods
4.1. Mineral Precipitation and Specimen Preparation
4.2. In Vitro Analysis of Ion Release and Cellular Reaction in Co-Culture
4.2.1. Ion Release and Mechanical Strength
4.2.2. Osteoblast/Osteoclast Co-Culture
4.2.3. Biochemical Analyses of Co-Culture, Confocal Laser Scanning Microscopy (cLSM) and Scanning Electron Microscopy (SEM)
4.3. Ethics Statement and Animal Study
4.4. µ-CT Analysis and ToF-SIMS
4.5. Sample Processing, Staining Procedures, and Histomorphometry
4.6. Immunohistochemistry
4.7. mRNA Preparation and Gene Expression Analysis
- (A)
- For new bone formation: 1. Alkaline phosphatase (ALP), an osteoblast marker indicating bone mineralization; 2. Osteocalcin (OCN), a noncollagenous protein secreted by osteoblasts, which plays a role in mineralization and calcium ion homeostasis; 3. Collagen type10 alpha1 (Col10α1), a hypertrophic chondrocytes marker; 4. Runt-related transcription factor 2 (Runx2), an essential protein for osteoblastic differentiation; 5. Collagen type I alpha1, a major component of type I collagen, (Col1α1).
- (B)
- For bone resorption: 1. TNFSF11gene (RANKL, RANK ligand) as a member of the tumor necrosis factor (TNF) cytokine family, ligand for osteoprotegerin, and a key factor, which regulates osteoclast differentiation and activation; 2. TNFRSF11B gene (osteoprotegerin; OPG), a decoy receptor for RANKL that works by neutralizing its function in osteoclastogenesis; 3. Carbonic anhydrase, an osteoclast marker involved in bone matrix dissolution. β2-microglobulin (B2M) was used as a reference gene. The primer pairs are provided in the Supplemental Table S2.
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cheung, W.H.; Miclau, T.; Chow, S.K.-H.; Yang, F.F.; Alt, V. Fracture healing in osteoporotic bone. Injury 2016, 47, 21–26. [Google Scholar] [CrossRef]
- Chen, C.-H.; Wang, L.; Serdar Tulu, U.; Arioka, M.; Moghim, M.M.; Salmon, B.; Chen, C.-T.; Hoffmann, W.; Gilgenbach, J.; Brunski, J.B.; et al. An osteopenic/osteoporotic phenotype delays alveolar bone repair. Bone 2018, 112, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Oheim, R.; Amling, M.; Ignatius, A.; Pogoda, P. Large animal model for osteoporosis in humans: The ewe. Eur. Cell. Mater. 2012, 24, 372–385. [Google Scholar] [CrossRef]
- Oheim, R.; Beil, F.T.; Köhne, T.; Wehner, T.; Barvencik, F.; Ignatius, A.; Amling, M.; Clarke, I.J.; Pogoda, P. Sheep model for osteoporosis: Sustainability and biomechanical relevance of low turnover osteoporosis induced by hypothalamic-pituitary disconnection. J. Orthop. Res. 2013, 31, 1067–1074. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, P.; Böcker, W.; El Khassawna, T.; Kampschulte, M.; Schlewitz, G.; Huerter, B.; Sommer, U.; Dürselen, L.; Ignatius, A.; Bauer, N.; et al. Bone matrix, cellularity, and structural changes in a rat model with high-turnover osteoporosis induced by combined ovariectomy and a multiple-deficient diet. Am. J. Pathol. 2014, 184, 765–777. [Google Scholar] [CrossRef]
- Shorr, E.; Carter, A.C. The usefulness of strontium as an adjuvant to calcium in the remineralization of the skeleton in man. Bull. Hosp. Joint Dis. 1952, 13, 59–66. [Google Scholar]
- Marie, P.J. Strontium as therapy for osteoporosis. Curr. Opin. Pharmacol. 2005, 5, 633–636. [Google Scholar] [CrossRef]
- Yang, F.; Yang, D.; Tu, J.; Zheng, Q.; Cai, L.; Wang, L. Strontium enhances osteogenic differentiation of mesenchymal stem cells and in vivo bone formation by activating Wnt/catenin signaling. Stem Cells 2011, 29, 981–991. [Google Scholar] [CrossRef]
- Nielsen, S.P. The biological role of strontium. Bone 2004, 35, 583–588. [Google Scholar] [CrossRef]
- Aimaiti, A.; Maimaitiyiming, A.; Boyong, X.; Aji, K.; Li, C.; Cui, L. Low-dose strontium stimulates osteogenesis but high-dose doses cause apoptosis in human adipose-derived stem cells via regulation of the ERK1/2 signaling pathway. Stem Cell Res. Ther. 2017, 8, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Kruppke, B.; Farack, J.; Wagner, A.-S.; Beckmann, S.; Heinemann, C.; Glenske, K.; Rößler, S.; Wiesmann, H.-P.; Wenisch, S.; Hanke, T. Gelatine modified monetite as a bone substitute material: An in vitro assessment of bone biocompatibility. Acta Biomater. 2016, 32, 275–285. [Google Scholar] [CrossRef]
- Kruppke, B.; Wagner, A.-S.; Rohnke, M.; Heinemann, C.; Kreschel, C.; Gebert, A.; Wiesmann, H.-P.; Mazurek, S.; Wenisch, S.; Hanke, T. Biomaterial based treatment of osteoclastic/osteoblastic cell imbalance—Gelatin-modified calcium/strontium phosphates. Mater. Sci. Eng. C 2019, 104, 109933. [Google Scholar] [CrossRef]
- Kruppke, B.; Heinemann, C.; Gebert, A.; Rohnke, M.; Weiß, M.; Henß, A.; Wiesmann, H.-P.; Hanke, T. Strontium substitution of gelatin modified calcium hydrogen phosphates as porous hard tissue substitutes. J. Biomed. Mater. Res. Part A 2020, 37057. [Google Scholar] [CrossRef]
- Peng, Z.; Tuukkanen, J.; Zhang, H.; Jämsä, T.; Väänänen, H.K. The mechanical strength of bone in different rat models of experimental osteoporosis. Bone 1994, 15, 523–532. [Google Scholar] [CrossRef]
- Wronski, T.J.; Lowry, P.L.; Walsh, C.C.; Ignaszewski, L.A. Skeletal alterations in ovariectomized rats. Calcif. Tissue Int. 1985, 37, 324–328. [Google Scholar] [CrossRef]
- Alt, V.; Thormann, U.; Ray, S.; Zahner, D.; Dürselen, L.; Lips, K.; El Khassawna, T.; Heiss, C.; Riedrich, A.; Schlewitz, G.; et al. A new metaphyseal bone defect model in osteoporotic rats to study biomaterials for the enhancement of bone healing in osteoporotic fractures. Acta Biomater. 2013, 9, 7035–7042. [Google Scholar] [CrossRef]
- Stuermer, E.K.; Sehmisch, S.; Rack, T.; Wenda, E.; Seidlova-Wuttke, D.; Tezval, M.; Wuttke, W.; Frosch, K.H.; Stuermer, K.M. Estrogen and raloxifene improve metaphyseal fracture healing in the early phase of osteoporosis. A new fracture-healing model at the tibia in rat. Langenbeck’s Arch. Surg. 2010, 395, 163–172. [Google Scholar] [CrossRef] [Green Version]
- Stürmer, E.K.; Seidlová-Wuttke, D.; Sehmisch, S.; Rack, T.; Wille, J.; Frosch, K.H.; Wuttke, W.; Stürmer, K.M. Standardized Bending and Breaking Test for the Normal and Osteoporotic Metaphyseal Tibias of the Rat: Effect of Estradiol, Testosterone, and Raloxifene. J. Bone Miner. Res. 2006, 21, 89–96. [Google Scholar] [CrossRef]
- Uusitalo, H.; Rantakokko, J.; Ahonen, M.; Jämsä, T.; Tuukkanen, J.; KäHäri, V.-M.; Vuorio, E.; Aro, H.T. A metaphyseal defect model of the femur for studies of murine bone healing. Bone 2001, 28, 423–429. [Google Scholar] [CrossRef]
- Monfoulet, L.; Rabier, B.; Chassande, O.; Fricain, J.-C. Drilled Hole Defects in Mouse Femur as Models of Intramembranous Cortical and Cancellous Bone Regeneration. Calcif. Tissue Int. 2010, 86, 72–81. [Google Scholar] [CrossRef]
- Claes, L.; Veeser, A.; Göckelmann, M.; Simon, U.; Ignatius, A. A novel model to study metaphyseal bone healing under defined biomechanical conditions. Arch. Orthop. Trauma Surg. 2009, 129, 923–928. [Google Scholar] [CrossRef]
- Heinemann, C.; Heinemann, S.; Worch, H.; Hanke, T.; Heinemann, C. Development of an osteoblast/osteoclast co-culture derived by human bone marrow stromal cells and human monocytes for biomaterials testing. Eur. Cell Mater. 2011, 21, 80–93. [Google Scholar] [CrossRef]
- Heinemann, C.; Heinemann, S.; Vater, C.; Worch, H.; Hanke, T. Influence of osteogenic supplements on the osteoclastogenesis of human monocytes. J. Dev. Biol. Tissue Eng. 2011, 3, 56–61. [Google Scholar]
- Kern, C.; Ray, S.; Gelinsky, M.; Bellew, A.T.; Pirkl, A.; Rohnke, M. New insights into ToF-SIMS imaging in osteoporotic bone research. Biointerphases 2020, 15, 031005. [Google Scholar] [CrossRef]
- Tamimi, F.; Sheikh, Z.; Barralet, J. Dicalcium phosphate cements: Brushite and monetite. Acta Biomater. 2012, 8, 474–487. [Google Scholar] [CrossRef]
- Fernández, E.; Gil, F.J.; Best, S.; Ginebra, M.P.; Driessens, F.C.; Planell, J.A. The cement setting reaction in the CaHPO4-alpha-Ca3(PO4)2 system: An X-ray diffraction study. J. Biomed Mater Res. 1998, 42, 403–406. [Google Scholar] [CrossRef]
- Kruppke, B.; Heinemann, C.; Wagner, A.-S.; Farack, J.; Wenisch, S.; Wiesmann, H.-P.; Hanke, T. Strontium ions promote in vitro human bone marrow stromal cell proliferation and differentiation in calcium-lacking media. Dev. Growth Differ. 2019, 61, 166–175. [Google Scholar] [CrossRef] [Green Version]
- Bonnelye, E.; Chabadel, A.; Saltel, F.; Jurdic, P. Dual effect of strontium ranelate: Stimulation of osteoblast differentiation and inhibition of osteoclast formation and resorption in vitro. Bone 2008, 42, 129–138. [Google Scholar] [CrossRef]
- Caudrillier, A.; Hurtel-Lemaire, A.-S.; Wattel, A.; Cournarie, F.; Godin, C.; Petit, L.; Petit, J.-P.; Terwilliger, E.; Kamel, S.; Brown, E.M.; et al. Strontium Ranelate Decreases Receptor Activator of Nuclear Factor-κB Ligand-Induced Osteoclastic Differentiation In Vitro: Involvement of the Calcium-Sensing Receptor. Mol. Pharmacol. 2010, 78, 569–576. [Google Scholar] [CrossRef]
- Hurtel-Lemaire, A.S.; Mentaverri, R.; Caudrillier, A.; Cournarie, F.; Wattel, A.; Kamel, S.; Terwilliger, E.F.; Brown, E.M.; Brazier, M. The calcium-sensing receptor is involved in Strontium ranelate-induced osteoclast apoptosis new insights into the associated signaling pathways. J. Biol. Chem. 2009, 284, 575–584. [Google Scholar] [CrossRef] [Green Version]
- Cianferotti, L.; Gomes, A.R.; Fabbri, S.; Tanini, A.; Brandi, M.L. The calcium-sensing receptor in bone metabolism: From bench to bedside and back. Osteoporos. Int. 2015, 26, 2055–2071. [Google Scholar] [CrossRef]
- Kern, C.; Quade, M.; Ray, S.; Thomas, J.; Schumacher, M.; Gemming, T.; Gelinsky, M.; Alt, V.; Rohnke, M. Investigation of strontium transport and strontium quantification in cortical rat bone by time-of-flight secondary ion mass spectrometry. J. R. Soc. Interface 2019, 16. [Google Scholar] [CrossRef] [Green Version]
- Rohnke, M.; Pfitzenreuter, S.; Mogwitz, B.; Henß, A.; Thomas, J.; Bieberstein, D.; Gemming, T.; Otto, S.K.; Ray, S.; Schumacher, M.; et al. Strontium release from Sr2+-loaded bone cements and dispersion in healthy and osteoporotic rat bone. J. Control. Release 2017, 262, 159–169. [Google Scholar] [CrossRef]
- Henrotin, Y.; Labasse, A.; Zheng, S.X.; Galais, P.; Tsouderos, Y.; Crielaard, J.M.; Reginster, J.Y. Strontium ranelate increases cartilage matrix formation. J. Bone Miner. Res. 2001, 16, 299–308. [Google Scholar] [CrossRef]
- Yu, D.; Ding, H.; Mao, Y.; Liu, M.; Yu, B.; Zhao, X.; Wang, X.; Li, Y.; Liu, G.; Nie, S.; et al. Strontium ranelate reduces cartilage degeneration and subchondral bone remodeling in rat osteoarthritis model. Acta Pharmacol. Sin. 2013, 34, 393–402. [Google Scholar] [CrossRef] [Green Version]
- Heinemann, S.; Heinemann, C.; Wenisch, S.; Alt, V.; Worch, H.; Hanke, T. Calcium phosphate phases integrated in silica/collagen nanocomposite xerogels enhance the bioactivity and ultimately manipulate the osteoblast/osteoclast ratio in a human co-culture model. Acta Biomater. 2013, 9, 4878–4888. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, C.; Heinemann, S.; Rößler, S.; Kruppke, B.; Wiesmann, H.-P.; Hanke, T. Organically modified hydroxyapatite (ormoHAP) nanospheres stimulate the differentiation of osteoblast and osteoclast precursors: A co-culture study. Biomed. Mater. 2019, 14, 035015. [Google Scholar] [CrossRef] [PubMed]
- Oswald, J.; Boxberger, S.; Jørgensen, B.; Feldmann, S.; Ehninger, G.; Bornhäuser, M.; Werner, C. Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells 2004, 22, 377–384. [Google Scholar] [CrossRef]
- Janckila, A.J.; Takahashi, K.; Sun, S.Z.; Yam, L.T. Tartrate-resistant acid phosphatase isoform 5b as serum marker for osteoclastic activity. Clin. Chem. 2001, 47, 74–80. [Google Scholar] [CrossRef] [Green Version]
- Ray, S.; Thormann, U.; Sommer, U.; El Khassawna, T.; Hundgeburth, M.; Henß, A.; Rohnke, M.; Lips, K.S.; Heiss, C.; Heinemann, S.; et al. Effects of macroporous, strontium loaded xerogel-scaffolds on new bone formation in critical-size metaphyseal fracture defects in ovariectomized rats. Injury 2016, 47 (Suppl. 1), 52–61. [Google Scholar] [CrossRef]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Müller, R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Miner. Res. 2010, 25, 1468–1486. [Google Scholar] [CrossRef] [PubMed]
- Henss, A.; Rohnke, M.; El Khassawna, T.; Govindarajan, P.; Schlewitz, G.; Heiss, C.; Janek, J. Applicability of ToF-SIMS for monitoring compositional changes in bone in a long-term animal model. J. R. Soc. Interface 2013, 10, 20130332. [Google Scholar] [CrossRef] [Green Version]
- Albers, J.; Schulze, J.; Beil, F.T.; Gebauer, M.; Baranowsky, A.; Keller, J.; Marshall, R.P.; Wintges, K.; Friedrich, F.W.; Priemel, M.; et al. Control of bone formation by the serpentine receptor Frizzled-9. J. Cell Biol. 2011, 192, 1057–1072. [Google Scholar] [CrossRef] [Green Version]
- Peters, A.; Toben, D.; Lienau, J.; Schell, H.; Bail, H.J.; Matziolis, G.; Duda, G.N.; Kaspar, K. Locally Applied Osteogenic Predifferentiated Progenitor Cells Are More Effective Than Undifferentiated Mesenchymal Stem Cells in the Treatment of Delayed Bone Healing. Tissue Eng. Part A 2009, 15, 2947–2954. [Google Scholar] [CrossRef]
- Henss, A.; Hild, A.; Rohnke, M.; Wenisch, S.; Janek, J. Time of flight secondary ion mass spectrometry of bone—Impact of sample preparation and measurement conditions. Biointerphases 2016, 11, 02A302. [Google Scholar] [CrossRef] [Green Version]
- Kokesch-Himmelreich, J.; Schumacher, M.; Rohnke, M.; Gelinsky, M.; Janek, J. ToF-SIMS analysis of osteoblast-like cells and their mineralized extracellular matrix on strontium enriched bone cements. Biointerphases 2013, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Sample Availability: Samples of the compounds PPGC + S 5:5 and PPGC + S 3:7 are available from the authors. |












| Tube Voltage /kVp | Tube Current/µA | Noise Reduction (Frame Averaging) | Rotation Steps/° | Projections | Isotropic Voxel Sidel Length/µm |
|---|---|---|---|---|---|
| 65 | 123 | 4-fold | 0.23 | 1440 | 8.93 |
| Figure 6 and Figure 7 | Empty Defect | PPGC + S 5:5 | PPGC + S 3:7 |
|---|---|---|---|
| Analysis Options | |||
| Cycle Time | 85 µs | 85 µs | 85 µs |
| Raster Mode | sawtooth | sawtooth | sawtooth |
| Primary Ion Current | 0.6 pA | 0.4 pA | 0.3 pA |
| Pixel density | 300 pixel/mm | 250 pixel/mm | 600 pixel/mm |
| Frame per Patch | 3 | 3 | 1 |
| Patch size | 0.4 mm | 0.4 mm | 0.5 mm |
| Primary Ion Shots/Frame/Pixel | 3 | 3 | 3 |
| Number of Scans | 3 | 3 | 6 |
| Primary Beam | ||
| Species | Ar8000+ | |
| Energy | /eV | 20,000 |
| Current | /pA | 100 |
| FoV | /µm² | 400 × 400 |
| Total Dose | 5 × 1011 | |
| Dose Density | /1/cm² | 3 × 1014 |
| Raster Mode | sawtooth | |
| Micro Raster Size | /pixel | 72 by 72 |
| Analysis options | ||
| Polarity | positive | |
| Cycle Time | /µs | 400 |
| Mode | Orbitrap Depth profile | |
| Cratersize | /µm² | 567.1 × 567.1 |
| Injection Time | /ms | 2950 |
| Duration | /s | 2.6 |
| Estimated Depth per Frame | /nm | 1.02 |
| Mass Resolution m/Δm (FWHM) | at m/z 70.07 (C4H8N+) | >425,000 |
| Mass Range | /m/z | 50–750 |
| Number of Scans | 400 scans |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kruppke, B.; Ray, S.; Alt, V.; Rohnke, M.; Kern, C.; Kampschulte, M.; Heinemann, C.; Budak, M.; Adam, J.; Döhner, N.; et al. Gelatin-Modified Calcium/Strontium Hydrogen Phosphates Stimulate Bone Regeneration in Osteoblast/Osteoclast Co-Culture and in Osteoporotic Rat Femur Defects—In Vitro to In Vivo Translation. Molecules 2020, 25, 5103. https://doi.org/10.3390/molecules25215103
Kruppke B, Ray S, Alt V, Rohnke M, Kern C, Kampschulte M, Heinemann C, Budak M, Adam J, Döhner N, et al. Gelatin-Modified Calcium/Strontium Hydrogen Phosphates Stimulate Bone Regeneration in Osteoblast/Osteoclast Co-Culture and in Osteoporotic Rat Femur Defects—In Vitro to In Vivo Translation. Molecules. 2020; 25(21):5103. https://doi.org/10.3390/molecules25215103
Chicago/Turabian StyleKruppke, Benjamin, Seemun Ray, Volker Alt, Marcus Rohnke, Christine Kern, Marian Kampschulte, Christiane Heinemann, Matthäus Budak, Josephine Adam, Nils Döhner, and et al. 2020. "Gelatin-Modified Calcium/Strontium Hydrogen Phosphates Stimulate Bone Regeneration in Osteoblast/Osteoclast Co-Culture and in Osteoporotic Rat Femur Defects—In Vitro to In Vivo Translation" Molecules 25, no. 21: 5103. https://doi.org/10.3390/molecules25215103
APA StyleKruppke, B., Ray, S., Alt, V., Rohnke, M., Kern, C., Kampschulte, M., Heinemann, C., Budak, M., Adam, J., Döhner, N., Franz-Forsthoffer, L., El Khassawna, T., Heiss, C., Hanke, T., & Thormann, U. (2020). Gelatin-Modified Calcium/Strontium Hydrogen Phosphates Stimulate Bone Regeneration in Osteoblast/Osteoclast Co-Culture and in Osteoporotic Rat Femur Defects—In Vitro to In Vivo Translation. Molecules, 25(21), 5103. https://doi.org/10.3390/molecules25215103