Pressureless and Low-Pressure Synthesis of Microporous Carbon Spheres Applied to CO2 Adsorption
Abstract
:1. Introduction
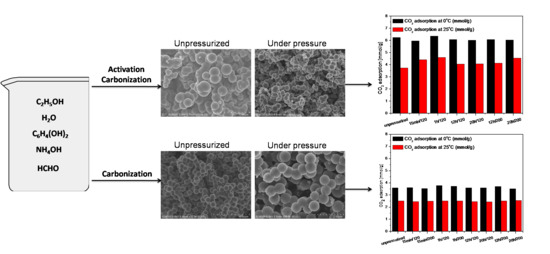
2. Results and Discussions
3. Materials Preparation
4. Materials Characterization
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- European Commission, Brussels, 11.12.2019 COM(2019) 640 Final. Available online: https://ec.europa.eu/info/sites/info/files/european-green-deal-communication_en.pdf (accessed on 2 September 2020).
- National Academies of Sciences, Engineering, and Medicine. In Negative Emissions Technologies and Reliable Sequestration: A Research Agenda; The National Academies Press: Washington, DC, USA, 2019.
- European Academies’ Science Advisory Council. In Negative Emission Technologies: What Role in Meeting Paris Agreement Targets? German National Academy of Sciences Leopoldina: Harley, Germany, 2018.
- Rifka, T.; Morosuk, T.; Tsatsaronis, G. Carbon capture and storage using low-temperature post-combustion technologies. Energy Sources Part A 2019. [Google Scholar] [CrossRef]
- Gómez-Díaz, D.; Muñiz-Mouro, A.; Navaza, J.M.; Rumbo, A. Diamine versus amines blend for CO2 chemical absorption. AIChE J. 2020. [Google Scholar] [CrossRef]
- Abd, A.A.; Naji, S.Z.; Hashim, A.S.; Othman, M.R. Carbon dioxide removal through physical adsorption using carbonaceous and non-carbonaceous adsorbents: A review. J. Environ. Chem. Eng. 2020, 8, 104142. [Google Scholar] [CrossRef]
- Rosli, A.; Ahmad, A.L.; Low, S.C. Enhancing membrane hydrophobicity using silica end-capped with organosilicon for CO2 absorption in membrane contactor. Sep. Purif. Technol. 2020, 251, 117429. [Google Scholar] [CrossRef]
- Gadipelli, S.; Howard, C.A.; Guo, J.; Skipper, N.T.; Zhang, H.; Shearing, P.R.; Brett, D.J.L. Superior multifunctional activity of nanoporous carbons with widely tunable porosity: Enhanced storage capacities for carbon-dioxide, hydrogen, water, and electric charge. Adv. Energy Mater. 2020, 10, 1903649. [Google Scholar] [CrossRef]
- D’Alessandro, D.M.; Smit, B.; Long, J.R. Carbon dioxide capture: Prospects for new materials. Angew. Chem. Int. Ed. 2010, 49, 6058–6082. [Google Scholar] [CrossRef] [Green Version]
- Staciwa, P.; Narkiewicz, U.; Sibera, D.; Moszyński, D.; Wróbel, R.J.; Cormia, R.D. Carbon spheres carbon spheres as CO2 sorbents. Appl. Sci. 2019, 9, 3349. [Google Scholar] [CrossRef] [Green Version]
- Khandaker, T.; Hossain, M.S.; Dhar, P.K.; Rahman, S.; Hossain, A.; Ahmed, M.B. Efficacies of carbon-based adsorbents for carbon dioxide capture. Processes 2020, 8, 654. [Google Scholar] [CrossRef]
- Qiao, W.; Song, Y.; Lim, S.; Hong, S.; Yoon, S.; Mochida, I.; Imaoka, T. Carbon nanospheres produced in an arc-discharge process. Carbon 2006, 44, 187–190. [Google Scholar] [CrossRef]
- Yang, S.; Zeng, H.; Zhao, H.; Zhang, H.; Cai, W. Luminescent hollow carbon shells and fullerene-like carbon spheres produced by laser ablation with toluene. J. Mater. Chem. 2011, 21, 4432–4436. [Google Scholar] [CrossRef]
- Choma, J.; Jamioła, D.; Augustynek, K.; Marszewski, M.; Gao, M.; Jaroniec, M. New opportunities in Stöber synthesis: Preparation of microporous and mesoporous carbon spheres. J. Mater. Chem. 2012, 22, 12636–12642. [Google Scholar] [CrossRef]
- Mi, Y.; Hu, W.; Dan, Y.; Liu, Y. Synthesis of carbon micro-spheres by a glucose hydrothermal method. Mater. Lett. 2008, 62, 1194–1196. [Google Scholar] [CrossRef]
- Yang, W.; Feng, Y.; Chu, W. Comparative study of textural characteristics on methane adsorption for carbon spheres produced by CO2 Activation. Int. J. Chem. Eng. 2014, 2014, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Rey-Raap, N.; Villanueva, S.F.; Menéndez, J.; Arenillas, A. Microporous carbon spheres derived from resorcinol-formaldehyde solutions. A new approach to coat supports. Microporous Mesoporous Mater. 2017, 252, 154–160. [Google Scholar] [CrossRef] [Green Version]
- Sibera, D.; Narkiewicz, U.; Kapica, J.; Serafin, J.; Michalkiewicz, B.; Wróbel, R.J.; Morawski, A.W. Preparation and characterisation of carbon spheres for carbon dioxide capture. J. Porous Mater. 2018, 26, 19–27. [Google Scholar] [CrossRef]
- Tian, H.; Liu, J.; O’Donnell, K.; Liu, T.; Liu, X.; Yan, Z.; Liu, S.; Jaroniec, M. Revisiting the Stöber method: Design of nitrogen-doped porous carbon spheres from molecular precursors of different chemical structures. J. Colloid Interface Sci. 2016, 476, 55–61. [Google Scholar] [CrossRef]
- Zhao, J.; Niu, W.; Zhang, L.; Cai, H.; Han, M.; Yuan, Y.; Majeed, S.; Anjum, S.; Xu, G. A Template-free and surfactant-free method for high-yield synthesis of highly monodisperse 3-aminophenol–formaldehyde resin and carbon nano/microspheres. Macromolecules 2012, 46, 140–145. [Google Scholar] [CrossRef]
- Wickramaratne, N.P.; Jaroniec, M. Activated carbon spheres for CO2 adsorption. ACS Appl. Mater. Interfaces 2013, 5, 1849–1855. [Google Scholar] [CrossRef]
- Liu, J.; Qiao, S.; Liu, H.; Chen, J.; Orpe, A.; Zhao, D.; Lu, G.Q. (Max) Extension of the stöber method to the preparation of monodisperse resorcinol-formaldehyde resin polymer and carbon spheres. Angew. Chem. Int. Ed. 2011, 50, 5947–5951. [Google Scholar] [CrossRef]
- Wickramaratne, N.P.; Xu, J.; Wang, M.; Zhu, L.; Dai, L.; Jaroniec, M. Nitrogen enriched porous carbon spheres: Attractive materials for supercapacitor electrodes and CO2 adsorption. Chem. Mater. 2014, 26, 2820–2828. [Google Scholar] [CrossRef]
- Ludwinowicz, J.; Jaroniec, M. Potassium salt-assisted synthesis of highly microporous carbon spheres for CO2 adsorption. Carbon 2015, 82, 297–303. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, J.; Xing, W.; Liu, B.; Zhang, J.; Lin, H.; Cui, H.; Zhuo, S. Resorcinol–formaldehyde resin-based porous carbon spheres with high CO2 capture capacities. J. Energy Chem. 2017, 26, 1007–1013. [Google Scholar] [CrossRef] [Green Version]
- Pari, G.; Darmawan, S.; Prihandoko, B. Porous carbon spheres from hydrothermal carbonization and KOH activation on Cassava and tapioca flour raw material. Procedia Environ. Sci. 2014, 20, 342–351. [Google Scholar] [CrossRef] [Green Version]
- Wickramaratne, N.P.; Jaroniec, M. Importance of small micropores in CO2capture by phenolic resin-based activated carbon spheres. J. Mater. Chem. A 2013, 1, 112–116. [Google Scholar] [CrossRef]
- Okunev, A.; Sharonov, V.; Aristov, Y.; Parmon, V. Sorption of carbon dioxide from wet gases by K2CO3-in-porous matrix: Influence of the matrix nature. React. Kinet. Catal. Lett. 2000, 71, 355–362. [Google Scholar] [CrossRef]
- Tripathi, N.K. Porous carbon spheres: Recent developments and applications. AIMS Mater. Sci. 2018, 5, 1016–1052. [Google Scholar] [CrossRef]
- Wang, Y.; Chang, B.; Guan, D.; Cheng, F. Mesoporous activated carbon spheres derived from resorcinol-formaldehyde resin with high performance for supercapacitors. J. Solid State Electrochem. 2015, 19, 1783–1791. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Park, S.-J. Determination of the optimal pore size for improved CO2 adsorption in activated carbon fibers. J. Colloid Interface Sci. 2013, 389, 230–235. [Google Scholar] [CrossRef]
- Casco, M.E.; Martínez-Escandell, M.; Silvestre-Albero, J.; Rodríguez-Reinoso, F. Effect of the porous structure in carbon materials for CO2 capture at atmospheric and high-pressure. Carbon 2014, 67, 230–235. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Watanabe, T.; Kanoh, H.; Hata, K.; Ohba, T. Cooperative CO2 adsorption promotes high CO2 adsorption density over wide optimal nanopore range. Adsorpt. Sci. Technol. 2017, 36, 625–639. [Google Scholar] [CrossRef] [Green Version]
- Staciwa, P.; Sibera, D.; Pełech, I.; Narkiewicz, U.; Łojkowski, W.; Dąbrowska, S.; Cormia, R. Effect of microwave assisted solvothermal process parameters on carbon dioxide adsorption properties of microporous carbon materials. Micropor. Mesopor. Mater. Unpublished work.
- Xu, S.; Liu, C.; Ye, F.; Guo, Y.; Wiezorek, J.M. Alkali-assisted hydrothermal route to control submicron-sized nanoporous carbon spheres with uniform distribution. Colloids Surf. A Physicochem. Eng. Asp. 2017, 515, 1–11. [Google Scholar] [CrossRef]
- Liu, X.; Song, P.; Hou, J.; Wang, B.; Xu, F.; Zhang, X. Revealing the dynamic formation process and mechanism of hollow carbon spheres: From bowl to sphere. ACS Sustain. Chem. Eng. 2018, 6, 2797–2805. [Google Scholar] [CrossRef]
- Kukulka, W.; Wenelska, K.; Baca, M.; Chen, X.; Mijowska, E. From hollow to solid carbon spheres: Time-dependent facile synthesis. Nanomaterials 2018, 8, 861. [Google Scholar] [CrossRef] [Green Version]
- Juhl, A.C.; Schneider, A.; Ufer, B.; Brezesinski, T.; Janek, J.; Fröba, M. Mesoporous hollow carbon spheres for lithium–sulfur batteries: Distribution of sulfur and electrochemical performance. Beilstein J. Nanotechnol. 2016, 7, 1229–1240. [Google Scholar] [CrossRef] [Green Version]
- Krishnamurthy, G.; Namitha, R. Synthesis of structurally novel carbon micro/nanospheres by low temperature-hydrothermal process. J. Chil. Chem. Soc. 2013, 58, 1930–1933. [Google Scholar] [CrossRef] [Green Version]
- Lima, A.M.F.; Musumeci, A.W.; Liu, H.-W.; Waclawik, E.R.; Silva, G.G. Purity evaluation and influence of carbon nanotube on carbon nanotube/graphite thermal stability. J. Therm. Anal. Calorim. 2009, 97, 257–263. [Google Scholar] [CrossRef]
- Long, J.W.; Laskoski, M.; Keller, T.M.; Pettigrew, K.A.; Zimmerman, T.N.; Qadri, S.B.; Peterson, G.W. Selective-combustion purification of bulk carbonaceous solids to produce graphitic nanostructures. Carbon 2010, 48, 501–508. [Google Scholar] [CrossRef]
- Datsyuk, V.; Kalyva, M.; Papagelis, K.; Parthenios, J.; Tasis, D.; Siokou, A.; Kallitsis, I.; Galiotis, C. Chemical oxidation of multiwalled carbon nanotubes. Carbon 2008, 46, 833–840. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Provisional). Pure Appl. Chem. 1982, 54, 2201–2218. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Musa, M.S.; Sanagi, M.M.; Nur, H.; Ibrahim, W.A.W. Understanding pore formation and structural deformation in carbon spheres during KOH activation. Sains Malays. 2015, 44, 613–618. [Google Scholar] [CrossRef]
- Heidarinejad, Z.; Dehghani, M.H.; Heidari, M.; Javedan, G.; Ali, I.; Sillanpää, M. Methods for preparation and activation of activated carbon: A review. Environ. Chem. Lett. 2020, 18, 393–415. [Google Scholar] [CrossRef]
- Liu, J.; Liu, X.; Sun, Y.; Sun, C.; Liu, H.; Stevens, L.A.; Li, K.; Snape, C.P. High density and super ultra-microporous-activated carbon macrospheres with high volumetric capacity for CO2 capture. Adv. Sustain. Syst. 2018, 2, 1700115–1700123. [Google Scholar] [CrossRef] [Green Version]
- Lendzion-Bieluń, Z.; Czekajło, Ł.; Sibera, D.; Moszyński, D.; Sreńscek-Nazzal, J.; Morawski, A.; Wrobel, R.J.; Michalkiewicz, B.; Arabczyk, W.; Narkiewicz, U. Surface characteristics of KOH-treated commercial carbons applied for CO2 adsorption. Adsorpt. Sci. Technol. 2017, 36, 478–492. [Google Scholar] [CrossRef] [Green Version]
- Serafin, J.; Narkiewicz, U.; Morawski, A.W.; Wróbel, R.J.; Michalkiewicz, B. Highly microporous activated carbons from biomass for CO2 capture and effective micropores at different conditions. J. CO2 Util. 2017, 18, 73–79. [Google Scholar] [CrossRef]
- Li, Y.; Li, D.; Rao, Y.; Zhao, X.; Wu, M. Superior CO2, CH4, and H2 uptakes over ultrahigh-surface-area carbon spheres prepared from sustainable biomass-derived char by CO2 activation. Carbon 2016, 105, 454–462. [Google Scholar] [CrossRef]
- Travis, W.; Gadipelli, S.; Guo, Z. Superior CO2 adsorption from waste coffee ground derived carbons. RSC Adv. 2015, 5, 29558–29562. [Google Scholar] [CrossRef]
- Presser, V.; McDonough, J.K.; Yeon, S.-H.; Gogotsi, Y. Effect of pore size on carbon dioxide sorption by carbide derived carbon. Energy Environ. Sci. 2011, 4, 3059–3066. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds used in the experiments are available from the authors. |










| Designation of the Sample | SBET | Total Pore Volume | CO2 Adsorption at 0 °C | CO2 Adsorption at 25 °C |
|---|---|---|---|---|
| (m2/g) | (cm3/g) | (mmol/g) | (mmol/g) | |
| The Samples without Addition of Potassium Oxalate | ||||
| RF | 472 | 0.25 | 3.59 | 2.52 |
| ARF_15 min/120 | 476 | 0.25 | 3.61 | 2.46 |
| ARF_15 min/200 | 478 | 0.26 | 3.53 | 2.49 |
| ARF_1 h/120 | 474 | 0.26 | 3.78 | 2.52 |
| ARF_1 h/200 | 483 | 0.27 | 3.73 | 2.52 |
| ARF_12 h/120 | 470 | 0.26 | 3.60 | 2.46 |
| ARF_20 h/120 | 486 | 0.28 | 3.59 | 2.44 |
| ARF_12 h/200 | 462 | 0.25 | 3.70 | 2.52 |
| ARF_20 h/200 | 473 | 0.29 | 3.51 | 2.55 |
| The Samples with Addition of Potassium Oxalate | ||||
| RF_7/1 | 904 | 0.49 | 6.25 | 3.74 |
| ARF_7/1_15 min/120 | 903 | 0.49 | 5.96 | 4.41 |
| ARF_7/1_1 h/120 | 969 | 0.51 | 6.35 | 4.60 |
| ARF_7/1_12 h/120 | 847 | 0.46 | 6.08 | 4.06 |
| ARF_7/1_20 h/120 | 831 | 0.45 | 6.02 | 4.08 |
| ARF_7/1_12 h/200 | 923 | 0.49 | 6.07 | 4.14 |
| ARF_7/1_20 h/200 | 986 | 0.54 | 6.03 | 4.55 |
| Carbon Precursor | CO2 Adsorption at 0 °C | CO2 Adsorption at 25 °C | Reference |
|---|---|---|---|
| Resorcinol-formaldehyde resin | 6.30 | 4.70 | [24] |
| Coal tar pitch | 6.00 | 4.03 | [47] |
| Fern leaves | 4.52 | 4.12 | [49] |
| Carrot peels | 5.64 | 4.18 | [49] |
| Starch | 4.40 | 3.40 | [50] |
| Pinecone biochar | 7.90 | [8] | |
| Waste coffee grounds | 7.50 | 4.21 | [51] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pełech, I.; Sibera, D.; Staciwa, P.; Narkiewicz, U.; Cormia, R. Pressureless and Low-Pressure Synthesis of Microporous Carbon Spheres Applied to CO2 Adsorption. Molecules 2020, 25, 5328. https://doi.org/10.3390/molecules25225328
Pełech I, Sibera D, Staciwa P, Narkiewicz U, Cormia R. Pressureless and Low-Pressure Synthesis of Microporous Carbon Spheres Applied to CO2 Adsorption. Molecules. 2020; 25(22):5328. https://doi.org/10.3390/molecules25225328
Chicago/Turabian StylePełech, Iwona, Daniel Sibera, Piotr Staciwa, Urszula Narkiewicz, and Robert Cormia. 2020. "Pressureless and Low-Pressure Synthesis of Microporous Carbon Spheres Applied to CO2 Adsorption" Molecules 25, no. 22: 5328. https://doi.org/10.3390/molecules25225328
APA StylePełech, I., Sibera, D., Staciwa, P., Narkiewicz, U., & Cormia, R. (2020). Pressureless and Low-Pressure Synthesis of Microporous Carbon Spheres Applied to CO2 Adsorption. Molecules, 25(22), 5328. https://doi.org/10.3390/molecules25225328