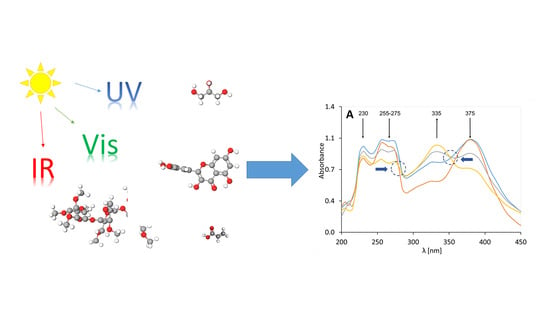
The Influence of UV Radiation on the Degradation of Pharmaceutical Formulations Containing Quercetin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. The UV Irradiation
2.2.2. Preparation
2.2.3. Calibration Curve
2.2.4. FTIR Studies
2.2.5. Spectrophotometric Method
2.2.6. Statistical Analysis
3. Results
3.1. Irradiated Substrates
3.2. Quercetin Ethanolic Solution, QA
3.3. PAA Gel with Glycerol and Quercetin, 2A
3.4. PAA Gel with Quercetin, 2B
3.5. MC Gel with Quercetin, 2C
3.6. Statistics
3.7. Student’s t Test
3.8. FT-IR
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hollósy, F. Effects of ultraviolet radiation on plant cells. Micron 2002, 33, 179–197. [Google Scholar] [CrossRef]
- Huang, X.X.; Bernerd, F.; Halliday, G.M. Ultraviolet A within sunlight induces mutations in the epidermal basal layer of engineered human skin. Am. J. Pathol. 2009, 174, 1534–1543. [Google Scholar] [CrossRef] [Green Version]
- Godar, D.E.; Landry, R.J.; Lucas, A.D. Increased UVA exposures and decreased cutaneous Vitamin D3 levels may be responsible for the increasing incidence of melanoma. Med. Hypotheses 2009, 72, 434–443. [Google Scholar] [CrossRef]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Ahmad, I.; Ahmed, S.; Anwar, Z.; Sheraz, M.A.; Sikorski, M. Photostability and photostabilization of drugs and drug products. Int. J. Photoenergy 2016, 2016, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Wichterle, O.; Lím, D. Hydrophilic Gels for Biological Use. Nat. Cell Biol. 1960, 185, 117–118. [Google Scholar] [CrossRef]
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Y.; Park, K. Environment-sensitive hydrogels for drug delivery. Adv. Drug Deliv. Rev. 2012, 64, 49–60. [Google Scholar] [CrossRef]
- Schwarz, T.W.; Levy, G.A. Report on the oxidative degradation of neutralized carbopol. J. Am. Pharm. Assoc. 1958, 47, 442–443. [Google Scholar] [CrossRef]
- Stability of Pharmaceutical Carbopol Polymer Gels Containing Hydrogen Peroxide; Lubrizol, Technical Data Sheet; Lubrizol Advanced Materials, Inc.: Cleveland, OH, USA, 2007; Volume TDS-361, pp. 1–5.
- Yousif, E.; Haddad, R. Photodegradation and photostabilization of polymers, especially polystyrene: Review. SpringerPlus 2013, 2, 398. [Google Scholar] [CrossRef] [Green Version]
- Deshpande, S.G.; Shirolkar, S. Sustained-release ophthalmic formulations of Pilocarpine. J. Pharm. Pharmacol. 1989, 41, 197–200. [Google Scholar] [CrossRef]
- Adams, I.; Davis, S.; Kershaw, R. Formulation of a Sterile Surgical Lubricant. J. Pharm. Pharmacol. 1972, 24, 178. [Google Scholar]
- Senna, M.; Helal, R.; Mostafa, T.; El-Naggar, A.W.M. Kinetic studies on the thermal decomposition of gamma-irradiated poly(vinyl alcohol (PVA)/methyl cellulose (MC) blends. J. Sci. Res. Sci. 2015, 32, 270–290. [Google Scholar] [CrossRef]
- Nan, W.; Ding, L.; Chen, H.; Khan, F.U.; Yu, L.; Sui, X.; Shi, X. Topical Use of Quercetin-Loaded Chitosan Nanoparticles Against Ultraviolet B Radiation. Front. Pharmacol. 2018, 9, 121–131. [Google Scholar] [CrossRef]
- Casagrande, R.; Georgetti, S.R.; Verri, W.A.; Dorta, D.J.; dos Santos, A.C.; Fonseca, M.J. Protective effect of topical formulations containing quercetin against UVB-induced oxidative stress in hairless mice. J. Photochem. Photobiol. B Biol. 2006, 84, 21–27. [Google Scholar] [CrossRef]
- Duan, Y. Ultraviolet-Visible spectrum characterizations of Quercetin in aqueous ethanol with different pH values. J. Chem. Pharm. Res. 2014, 6, 236–240. [Google Scholar]
- Danish, M.; Mumtaz, M.W.; Fakhar, M.; Rashid, U. Response Surface Methodology: An Imperative Tool for the Optimized Purification of the Residual Glycerol from Biodiesel Production Process. Chiang Mai J. Sci. 2016, 43, 1–13. [Google Scholar]
- Kataoka, Y.; Kitadai, N.; Hisatomi, O.; Nakashima, S. Nature of hydrogen bonding of water molecules in aqueous solutions of glycerol by attenuated total reflection (ATR) Infrared Spectroscopy. Appl. Spectrosc. 2011, 65, 436–441. [Google Scholar] [CrossRef]
- Catauro, M.; Papale, F.; Bollino, F.; Piccolella, S.; Marciano, S.; Nocera, P.; Pacifico, S.; Bollino, F. Silica/quercetin sol–gel hybrids as antioxidant dental implant materials. Sci. Technol. Adv. Mater. 2015, 16, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Dubinsky, S.; Grader, G.S.; Shter, G.E.; Silverstein, M.S. Thermal degradation of poly(acrylic acid) containing copper nitrate. Polym. Degrad. Stab. 2004, 86, 171–178. [Google Scholar] [CrossRef]
- Kaczmarek, H.; Szalla, A. Photochemical transformation in poly(acrylic acid)/poly(ethylene oxide) complexes. J. Photochem. Photobiol. A 2006, 180, 46–53. [Google Scholar] [CrossRef]
- Karrasch, A.; Jäger, C.; Saake, B.; Potthast, A.; Rosenau, T. Solid-state NMR studies of methyl celluloses. Part 2: Determination of degree of substitution and O-6 vs. O-2/O-3 substituent distribution in commercial methyl cellulose samples. Cellulose 2009, 16, 1159–1166. [Google Scholar] [CrossRef]
- Nadour, M.; Boukraa, F.; Ouradi, A.; Benaboura, A. Effects of methylcellulose on the properties and morphology of polysulfone membranes prepared by phase inversion. Mater. Res. 2017, 20, 339–348. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Liu, D.; Liu, R.H. Sodium borohydride/chloranil-based assay for quantifying total flavonoids. J. Agric. Food Chem. 2008, 56, 9337–9344. [Google Scholar] [CrossRef]
- Ross, J.A.; Kasum, C.M. Dietary flavonoids: Bioavailability, metabolic effects, and safety. Annu. Rev. Nutr. 2002, 22, 19–34. [Google Scholar] [CrossRef]
- Zvezdanović, J.; Stanojević, J.; Marković, D.; Cvetković, D. Irreversible UV-induced quercetin and rutin degradation in solution studied by UV spectrophotometry and HPLC chromatography. J. Serbian Chem. Soc. 2012, 77, 297–312. [Google Scholar] [CrossRef]
- MolView.Org. Available online: http://molview.org/ (accessed on 1 July 2019).
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Fahlman, B.M.; Krol, E.S. UVA and UVB radiation-induced oxidation products of quercetin. J. Photochem. Photobiol. B Biol. 2009, 97, 123–131. [Google Scholar] [CrossRef]
- Chaaban, H.; Ioannou, I.; Paris, C.; Charbonnel, C.; Ghoul, M. The photostability of flavanones, flavonols and flavones and evolution of their antioxidant activity. J. Photochem. Photobiol. A Chem. 2017, 336, 131–139. [Google Scholar] [CrossRef]
- Vincentini, F.T.M.C.; Georgetti, R.S.; Jabor, J.R.; Fonseca, M.V.; Caris, J.A.; Vitória, M.; Bentley, M.V.L.B.; Maria, J.V.; Fonseca, M.V. Photostability of quercetin under exposure to UV irradiation. Lat. Am. J. Pharm. 2007, 26, 119–124. [Google Scholar]
- Scalia, S.; Mezzena, M. Incorporation of quercetin in lipid microparticles: Effect on photo- and chemical-stability. J. Pharm. Biomed. Anal. 2009, 49, 90–94. [Google Scholar] [CrossRef]
- Smith, G.J.; Thomsen, S.J.; Markham, K.R.; Andary, C.; Cardon, D. The photostabilities of naturally occurring 5-hydroxyflavones, flavonols, their glycosides and their aluminium complexes. J. Photochem. Photobiol. A Chem. 2000, 136, 87–91. [Google Scholar] [CrossRef]
- Dall’Acqua, S.; Miolo, G.; Innocenti, G.; Caffieri, S. The photodergradation of quercetin: Relation to oxidation. Molecules 2012, 17, 8898–8907. [Google Scholar] [CrossRef] [Green Version]
- Russo, A.; Acquaviva, R.; Campisi, A.; Sorrenti, V.; Di Giacomo, C.; Virgata, G.; Barcellona, M.L.; Vanella, A. Bioflavonoids as antiradicals, antioxidants and DNA cleavage protectors. Cell Biol. Toxicol. 2000, 16, 91–98. [Google Scholar] [CrossRef]
- Falkovskaia, E.; Sengupta, P.; Kasha, M. Photophysical induction of dual fluorescence of quercetin and related hydroxyflavones upon intermolecular H-bonding to solvent matrix. Chem. Phys. Lett. 1998, 297, 109–114. [Google Scholar] [CrossRef]
- Choquenet, B.; Couteau, C.; Paparis, E.; Coiffard, L. Quercetin and Rutin as Potential Sunscreen Agents: Determination of Efficacy by an in Vitro Method. J. Nat. Prod. 2008, 71, 1117–1118. [Google Scholar] [CrossRef]
- Sokolová, R.; Ramešová, Š.; Degano, I.; Hromadová, M.; Gál, M.; Žabka, J. The oxidation of natural flavonoid quercetin. Chem. Commun. 2012, 48, 3433–3435. [Google Scholar] [CrossRef]
- Jerzykiewicz, M.; Cwielag, I.; Jerzykiewicz, W.; Ćwieląg-Piasecka, I. The antioxidant and anticorrosive properties of crude glycerol fraction from biodiesel production. J. Chem. Technol. Biotechnol. 2009, 84, 1196–1201. [Google Scholar] [CrossRef]












| Sample Acronym | PA [g] | MC [g] | NaOH [g] | Glycerol [g] | Aqua [g] | QA ** [mg] |
|---|---|---|---|---|---|---|
| Gel 1A | 0.3 | - | q.s. * | 25.0 | 73.7 | - |
| Gel 1B | 0.3 | - | q.s. * | - | 94.7 | - |
| Gel 1C | - | 4 | - | - | 96.0 | - |
| Preparation 2A | 0.3 | - | q.s. * | 25.0 | 73.7 | 0.2 |
| Preparation 2B | 0.3 | - | q.s. * | - | 94.7 | 0.2 |
| Preparation 2C | - | 4 | - | - | 96.0 | 0.2 |
| Preparation | 2A * | 2B * | 2C * | QA ** |
|---|---|---|---|---|
| slope factor a | 559.36 | 833.30 | 596.80 | 24406 |
| standard error for a | 19.48 | 115.14 | 85.63 | 368.1 |
| coefficient b | 0.092 | 0.091 | 0.333 | −0.017 |
| standard error for b | 0.028 | 0.111 | 0.094 | 0.010 |
| linear correlation coefficient | 0.994 | 0.929 | 0.924 | 0.998 |
| Parameter | Preparation 2A | Preparation 2B | Preparation 2C | QA *** | ||||
|---|---|---|---|---|---|---|---|---|
| Irradiated | Unexposed | Irradiated | Unexposed | Irradiated | Unexposed | Irradiated | Unexposed | |
| slope factor a | −1.952·10−3 | −2.406·10−4 | −5.03210−4 | −4.241·10−5 | −1.679·10−3 | −8.950·10−4 | −1.404·10−4 | −1.586·10−5 |
| coefficient b | 1.176 | 1.171 | 7.664·10−1 | 7.656·10−1 | 1.331 | 1.256 | 9.752·10−1 | 9.874·10−1 |
| standard error a | 1.974·10−5 | 3.157·10−5 | 2.966·10−5 | 5.149·10−5 | 6.138·10−5 | 1.440·10−4 | 2.159·10−5 | 1.358·10−5 |
| standard error b | 2.678·10−3 | 4.282·10−3 | 4.023·10−3 | 6.985·10−3 | 8.326·10−3 | 1.954·10−2 | 2.928·10−3 | 1.842·10−3 |
| linear correlation coefficient | 9.991·10−1 | 8.658·10−1 | 9.697·10−1 | 7.009·10−2 | 9.881·10−1 | 8.110·10−1 | 8.246·10−1 | 1.317·10−1 |
| standard error of y estimation | 4.141·10−3 | 6.622·10−3 | 6.221·10−3 | 1.080·10−2 | 1.287·10−2 | 3.022·10−2 | 4.528·10−3 | 2.848·10−3 |
| parameter F | 9.783·103 | 5.808·101 | 2.878·102 | 6.783·10−1 | 7.482·102 | 3.860·101 | 4.232·101 | 1.365·100 |
| p | <0.05 | <0.05 | <0.05 | 4.314·10−1 | <0.05 | <0.05 | <0.05 | 2.727·10−1 |
| Parameter | Preparation 2A | Preparation 2B | Preparation 2C | QA *** |
|---|---|---|---|---|
| t | 4.598·101 | 7.754·100 | 1.072·101 | 5.506·100 |
| p | <0.0001 | <0.0001 | <0.0001 | 3.770·10−4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golonka, I.; Wilk, S.; Musiał, W. The Influence of UV Radiation on the Degradation of Pharmaceutical Formulations Containing Quercetin. Molecules 2020, 25, 5454. https://doi.org/10.3390/molecules25225454
Golonka I, Wilk S, Musiał W. The Influence of UV Radiation on the Degradation of Pharmaceutical Formulations Containing Quercetin. Molecules. 2020; 25(22):5454. https://doi.org/10.3390/molecules25225454
Chicago/Turabian StyleGolonka, Iwona, Stanisław Wilk, and Witold Musiał. 2020. "The Influence of UV Radiation on the Degradation of Pharmaceutical Formulations Containing Quercetin" Molecules 25, no. 22: 5454. https://doi.org/10.3390/molecules25225454
APA StyleGolonka, I., Wilk, S., & Musiał, W. (2020). The Influence of UV Radiation on the Degradation of Pharmaceutical Formulations Containing Quercetin. Molecules, 25(22), 5454. https://doi.org/10.3390/molecules25225454