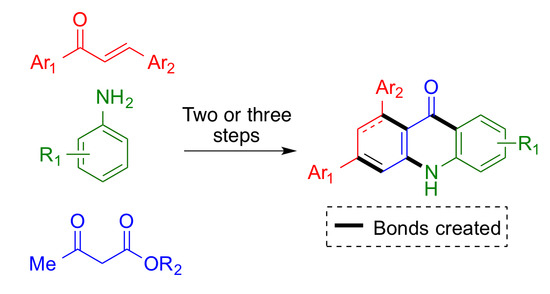
3. Materials and Methods
3.1. General Experimental Information
All reagents and solvents were of commercial quality and were used as received. Reactions were monitored by TLC analysis, on Merck silica gel-G aluminum plates with fluorescent indicator. Melting points were measured in open capillary tubes and are uncorrected. A CEM Discover microwave synthesizer with microwave power maximum level of 300 W and microwave frequency of 2455 MHz was employed for the microwave-assisted reactions. The 1H-NMR, 13C-NMR and CH-correlation spectra were recorded on a Bruker (Avance) 250 MHz or 500 MHz NMR instrument maintained by the CAI de Resonancia Magnética Nuclear, Universidad Complutense, using CDCl3, d6-DMSO or CD3OD as solvents and residual non-deuterated solvents as internal standards. Topspin (Bruker) or Mestrenova (Mestrelab) software packages were used throughout for data processing; chemical shifts are given in parts per million (δ-scale) and coupling constants are given in Hertz. Subjective 13C-NMR assignments are based on 2d_NMR experiments for representative compounds, summarized in the Supporting Information. Combustion microanalyses were performed by the CAI de Microanálisis Elemental, Universidad Complutense, on a Leco 932 CHNS analyzer. IR spectra were recorded on a Perkin Elmer Paragon 1000 FT-IR instrument using thin films placed on a KBr disk, which were obtained by evaporation of organic solvent solution of the compounds.
3.2. General Procedure for the Synthesis of 2,4-Diaryl-2,3-dihydroanthranilates 1
To a stirred solution of ethyl acetoacetate (311.0 to 974.8 mg, 2.39 to 7.49 mmol) and aniline (281.3 to 906.8 mg, 3.02 to 9.74 mmol, 1.3 eq) in ethanol (5 mL) was added CAN (65.5 mg, 0.12 mmol, 5 mol%). Stirring was continued for 30 min at room temperature. The appropriate chalcone (730 mg to 2.0 g, 2.63 to 8.24 mmol, 1.1 eq) was then added to the stirred solution and the mixture was heated under reflux for 8 h. After completion of the reaction, as indicated by TLC, the mixture was dissolved in ether (30 mL), washed with water, brine, dried (anhydrous Na
2SO
4) and the solvent was evaporated under reduced pressure. The final products were purified by flash silica column chromatography eluting with a petroleum ether-ethyl acetate mixture (9/1,
v/
v). Compounds
1a–
k were known in the literature [
26]. Characterization data for new compounds are given below (see
Supplementary Materials). Compound numbering used in the assignment of
13C-NMR signals is also given.
![Molecules 25 05565 i010]()
Ethyl 4″-methoxy-5′-(phenylamino)-2′,3′-dihydro-[1,1′:3′,1″-terphenyl]-4″-carboxylate (1l). Prepared from 1.78 g (7.45 mmol) of the corresponding chalcone. Yield: 2.7 g (6.26 mmol, 84%) as a yellow solid. Mp 105 °C. IR (cm−1): 3237, 3053, 2984, 2108, 1736, 1639. 1H-NMR (250 MHz, CDCl3) δ 10.64 (s, 1H), 7.27–7.15 (m, 6H), 7.12–7.07 (m, 2H), 7.06–6.99 (m, 3H), 6.70–6.62 (m, 2H), 6.56 (d, J = 2.8 Hz, 2H), 4.21 (dd, J = 8.5, 1.7 Hz, 1H), 4.03 (m, 2H), 3.64 (s, 3H), 3.11 (ddd, J = 16.6, 8.4, 2.9 Hz, 1H), 2.87 (dd, J = 16.6, 1.7 Hz, 1H), 1.10 (t, J = 7.1 Hz, 3H). 13C-NMR (63 MHz, CDCl3) δ 170.3 (CO), 156.0 (C-4″), 151.0 (C-5′), 144.1 (C-1″′), 140.0 (C-1′), 140.0 (C-1), 137.1 (C1″), 129.2 (C-5″′ and 3″′), 128.6 (C-3 and 5), 128.5 (C-2″ and C-6″), 128.3 (C-4), 125.9 (C-6 and C-2), 123.8 (C-4″′), 123.2, (C-6″′ and 2″′)118.0 (C6′), 113.5 (C-3″and C-5″), 95.7 (C-4′), 59.5(CH3-CH2O), 55.2 (MeO), 36.0 (C-3′), 34.8 (C-2″), 14.5 (CH3-CH2O). Anal. Calc. for C28H27O3N: C, 79.03; H, 6.40; N, 3.29. Found C, 78.83; H, 6.18; N, 3.09.
Ethyl 4″-chloro-5′-(phenylamino)-2′,3′-dihydro-[1,1′:3′,1″-terphenyl]-4′-carboxylate (1m). Prepared from 2.0 g (8.24 mmol) of the corresponding chalcone. Yield: 2.7 g (6.26 mmol, 76%) as a yellow solid. Mp: 120 °C. IR (cm−1): 3224, 3038, 2974, 2098, 1734, 1640. 1H-NMR (250 MHz, CDCl3) δ 10.76 (s, 1H), 7.38–7.06 (m, 14H), 6.64 (d, J = 2.8 Hz, 1H), 4.35–4.26 (m, 1H), 4.22–3.98 (m, 2H), 3.21 (ddd, J = 16.7, 8.6, 2.9 Hz, 1H), 2.92 (dd, J = 16.7, 1.7 Hz, 1H), 1.16 (t, J = 7.1 Hz, 3H). 13C-NMR (63 MHz, CDCl3) δ 170.2 (CO), 151.5 (C-5′), 144.0 (C-1″′), 143.7 (C-1″), 139.8 (C-1′), 131.8 (C-1), 129.3 (C-4″), 128.9 (C-5″′ and 3″′), 128.8 (C-2″ and C-6″), 128.8 (C-3″ and C-5″), 128.4 (C-3 and 5), 126.0 (C-2, C-4 and C-6), 124.1 (C-4″′), 123.4 (C-2″′ and 6″′)), 118.1 (C-6′), 94.7 (C4′), 59.6 (CH3-CH2O), 36.5 (C-3′), 34.6 (C-2′), 14.6 (CH3-CH2O). Anal. Calc. for C27H24O2NCl C, 75.43; H, 5.63; N, 3.26. Found C, 75.39; H, 5.54; N, 3.26.
Ethyl 2″,4,4″-trimethoxy-5′-(phenylamino)-2′,3′-dihydro-[1,1′:3′,1″-terphenyl]-4′-carboxylate (1n). Prepared from 1.0 g (3.35 mmol) of the corresponding chalcone. Yield: 1.1 g (2.31 mmol, 69%) as a pale yellow solid (Mp: 110 °C. IR (cm−1): 3260, 2952, 2838, 2115, 1737, 1640.1H-NMR (250 MHz, CDCl3) δ 10.82 (s, 1H), 7.35 (dd, J = 8.4, 7.2 Hz, 2H), 7.28–7.16 (m, 4H), 7.11 (d, J = 7.3 Hz, 1H), 6.92 (dd, J = 8.0, 0.7 Hz, 1H), 6.83–6.77 (m, 2H), 6.58 (d, J = 2.9 Hz, 1H), 6.39 (d, J = 8.2 Hz, 2H), 4.25 (dt, J = 8.0, 1.2 Hz, 1H), 4.22–4.04 (m, 2H), 3.79 (s, 6H), 3.70 (s, 3H), 3.21 (ddd, J = 16.6, 8.3, 2.9 Hz, 1H), 2.87 (dd, J = 16.7, 1.7 Hz, 1H), 1.22 (t, J = 7.1 Hz, 3H). 13C-NMR (63 MHz, CDCl3) δ 170.7 (CO), 161.2 (C-4), 158.7 (C-4″), 158.1 (C-6″), 151.8 (C5′), 143.7 (C-1″′), 140.4 (C-1′), 137.8, 130.0 (C1, C1′, C1″ or C1″′), 129.3 (C-6, 2, 5″′ and 2″′), 128.8 (C-1 and C-2″), 123.7 (C-4″′), 123.6 (C-2″′ and 6″′), 123.3 (C-1″), 119.9 (C-6′), 113.6 (C-3 and C-5), 104.6 (C-3″), 99.2 (C-5″), 95.3 (C4′), 59.6 (CH3-CH2O), 55.8 (MeO), 55.7 (MeO), 55.6 (MeO), 36.9 (C-3′), 36.5 (C-2′) 14.5 (CH3-CH2O). Anal. Calc. for C30H31O5N C, 74.21; H, 6.44; N, 2.88. Found C, 73.89; H, 6.24; N, 2.90.
Ethyl 4,4″-dichloro-5′-(phenylamino)-2′,3′-dihydro-[1,1′:3′,1″-terphenyl]-4′-carboxylate (1o). Prepared from 730 mg (2.63 mmol) of the corresponding chalcone. Yield: 720 mg (1.55 mmol, 59%) as a yellow solid. Mp: 142 °C. IR (cm−1): 3219, 3056, 2976, 1898, 2099, 1639. 1H-NMR (250 MHz, CDCl3) δ 10.83 (s, 1H), 7.49–7.15 (m, 13H), 6.70 (d, J = 2.8 Hz, 1H), 4.38 (dd, J = 8.5, 1.7 Hz, 1H), 4.30–4.10 (m, 2H), 3.28 (ddd, J = 16.6, 8.5, 2.9 Hz, 1H), 2.95 (dd, J = 16.6, 1.8 Hz, 1H), 1.26 (t, J = 7.1 Hz, 3H). 13C-NMR (63 MHz, CDCl3) δ 170.3 (CO), 151.4 (C-5′), 143.7 (C-1″′), 142.9 (C-1″), 139.9 (C-1′), 138.4 (C-1), 134.9 (C-4), 132.2 (C-4″), 129.6 (C-3″′ and C-5″′), 129.2 (C-2″ and 6″), 129.0 (C-3 and 5), 128.6 (C-3″ and 5″), 127.4 (C-2 and C-6), 124.5 (C-4″′), 123.6 (C-2″′ and 6″′), 118.7 (C-6′), 95.1 (C-4′), 59.9 (CH3-CH2O), 36.7 (C-2′), 34.8 (C-3′), 14.8 (CH3-CH2O). Anal. Calc. for C27H23O2NCl2 C, 69.83; H, 4.99; N, 3.02. Found C, 69.51; H, 4.86; N, 3.02.
3.3. General Procedure for the Synthesis of 1,3-Diaryl-1,2-dihydroacridin-9(10H)-ones 2
A microwave tube containing a solution of the suitable compound 1 (150 to 474 mg, 0.3 to 1.0 mmol) in dimethylformamide (3 mL), was closed and placed in the cavity of a CEM Discover focused microwave oven. The reaction mixture was heated with a maximum power of 200 W and a temperature gradient was programmed to achieve 250 °C starting from room temperature over 5 min. Then temperature was kept constant at 250 °C, by microwave irradiated for 90 min. The reaction mixture was cooled to room temperature and the solvent was removed under reduced pressure. The crude mixture was washed with cool chloroform (3 mL) and the precipitate obtained was filtered to obtain the desired product. Compound 2h was not obtained and its dehydrogenation derivative 3h was isolated instead. Compound numbering used in the assignment of 13C-NMR signals is given below.
![Molecules 25 05565 i011]()
1,3-Diphenyl-1,2-dihydroacridin-9(10H)-one (2a). Prepared from compound 1a (396 mg, 1.0 mmol). Yield: 328 mg (0.94 mmol, 94%); pale yellow solid. Mp: 287–288 °C. IR νmax (film): 3064, 3027, 2874, 1623, 1572, 1541 cm−1. 1H-NMR (250 MHz, DMSO-d6) δ 11.83 (s, 1H), 8.06 (dd, J = 8.1, 1.1 Hz, 1H), 7.68–7.60 (m, 1H), 7.59–7.52 (m, 3H), 7.48–7.36 (m, 3H), 7.33–7.23 (m, 2H), 7.23–7.14 (m, 3H), 7.14–7.07 (m, 1H), 6.89 (d, J = 2.6 Hz, 1H), 4.61 (d, J = 7.7 Hz, 1H), 3.25 (ddd, J = 17.7, 7.7, 2.6 Hz, 1H), 3.08 (dd, J = 17.4, 1.3 Hz, 1H). 13C-NMR (63 MHz, DMSO-d6) δ 174.3 (CO), 145.3 (C-15), 144.5 (C-19), 143.6 (C-3), 139.3(C-12), 138.8 (C-19), 131.4 (C-9), 129.1 (C-22), 128.9 (C-21), 128.6 (C-17), 127.1 (C-16), 126.1 (C-18), 125.5 (C-20), 125.1 (C-7), 125.0 (C-11), 122.8 (C-10), 118.0 (C-8), 117.2 (C-4), 113.7 (C-14), 34.0 (C-1), 33.8 (C-2). Anal. Calc. for C25H19NO (M = 349.42): C, 85.93; H, 5.48; N, 4.01; found: C, 85.96; H, 5.52; N, 4.07.
7-(Dimethylamino)-1,3-diphenyl-1,2-dihydroacridin-9(10H)-one (2b). Prepared from compound 1b (439 mg, 1.0 mmol). Yield: 377 mg, (0.96 mmol, 96%); yellow solid. Mp: 299–300 °C. IR νmax (film): 2863, 2788, 1612, 1566, 1473 cm−1. 1H-NMR (500 MHz, DMSO-d6) δ 11.67 (s, 1H), 7.55 (d, J = 7.2 Hz, 2H), 7.46 (d, J = 9.1 Hz, 1H), 7.42 (t, J = 7.4 Hz, 2H), 7.40–7.34 (m, 1H), 7.27 (dd, J = 9.1, 2.9 Hz, 1H), 7.22 (d, J = 7.3 Hz, 3H), 7.14 (t, J = 7.5 Hz, 2H), 7.11–7.05 (m, 1H), 6.86 (d, J = 2.7 Hz, 1H), 4.63 (d, J = 8.2 Hz, 1H), 3.25 (ddd, J = 17.1, 8.4, 2.7 Hz, 1H), 3.07 (d, J = 16.3 Hz, 1H), 2.94 (s, 6H). 13C-NMR (126 MHz, DMSO-d6) δ 174.2 (CO), 147.7 (C-7), 145.8 (C-15), 145.0 (C-19), 142.8 C10, 139.8 (C-14), 132.2 (C-3), 129.7 (C-22), 129.7 (C-21), 128.8 (C-17), 127.9 (C-16), 127.1 (C-18), 126.7 (C-20), 126.2 (C-7), 120.2 (C-9), 119.8 (C-8), 118.3 (C-4), 113.0(C-12), 105.4 (C-11), 41.4 (Me2N), 34.8(C-1), 34.8 (C-2). Anal. Calc. for C27H24N2O (M = 392.49): C, 82.62; H, 6.16; N, 7.14; found: C, 82.59; H, 6.13; N, 7.17.
7-Fluoro-1,3-diphenyl-1,2-dihydroacridin-9(10H)-one (2c). Prepared from compound 1c (414 mg, 1.0 mmol). Yield: 316 mg (0.86 mmol, 86%); pale yellow solid. Mp: 294–295 °C. IR νmax (film): 2939, 2856, 2647, 1628, 1581 cm−1. 1H-NMR (250 MHz, DMSO-d6) δ 11.99 (s, 1H), 7.72 (dd, J = 9.5, 2.8 Hz, 1H), 7.68–7.51 (m, 5H), 7.49–7.37 (m, 3H), 7.26–7.16 (m, 2H), 7.15–7.08 (m, 2H), 6.90 (d, J = 2.6 Hz, 1H), 4.62 (d, J = 7.5 Hz, 1H), 3.33–3.23 (m, 1H), 3.10 (dd, J = 17.4, 1.3 Hz, 1H). 13C-NMR (63 MHz, DMSO-d6) δ 173.5 (CO), 158.2 (d, J = 484.0 Hz) (C-10), 145.7 (C-5), 144.3 (C-7), 143.8 (C-15), 138.7 (C-19), 136.0 (C-3), 129.2 (C-12), 128.9 (C-21), 128.1(C-22), 127.0 (C-17), 126.2 (C-20), 126.1 (C-16), 125.5 (C-18), 120.6 (d, J = 51.6 Hz) (C-9), 120.0 (C-8), 117.0 (C-4), 113.1 (C-14), 109.0 (d, J = 50.4 Hz) (C-11), 33.9(C-1), 33.8 (C-2). Anal. Calc. for C25H18FNO (M = 367.41): C, 81.72; H, 4.94; N, 3.81; found: C, 81.68; H, 4.93; N, 3.76.
7-Chloro-1,3-diphenyl-1,2-dihydroacridin-9(10H)-one (2d). Prepared from compound 1d (430 mg, 1.0 mmol). Yield: 288 mg, (0.75 mmol, 75%); pale yellow solid. Mp: 275–276 °C. IR νmax (film): 3060, 2923, 2887, 1620, 1542 cm−1. 1H-NMR (250 MHz, DMSO-d6) δ 12.01 (s, 1H), 7.99 (d, J = 2.3 Hz, 1H), 7.67 (dd, J = 8.8, 2.4 Hz, 1H), 7.62–7.54 (m, 3H), 7.49–7.35 (m, 4H), 7.20 (dd, J = 10.5, 7.4 Hz, 4H), 7.14–7.08 (m, 1H), 6.88 (d, J = 2.5 Hz, 1H), 4.58 (d, J = 7.5 Hz, 1H), 3.27 (d, J = 8.3 Hz, 1H), 3.09 (d, J = 16.5 Hz, 1H). 13C-NMR (63 MHz, DMSO-d6) δ 173.1 (CO), 146.0 (C-5), 144.2 (C-7), 138.7 (C-15), 137.9 (C-19), 131.5 (C-17), 129.2 (C-12), 129.0 (C-3), 128.1 (C-9), 127.4 (C-22), 127.0 (C-21), 126.1 (C-17), 126.0 (C-16), 125.6 (C-18), 124.0 (C-20), 122.8 (C-11), 120.5 (C-8), 117.0 (C4), 114.1 (C14), 33.9 (C1), 33.8 (C2). Anal. Calc. for C25H18ClNO (M = 383.87): C, 78.22; H, 4.73; N, 3.65; found: C, 78.18; H, 4.75; N, 3.61.
7-Bromo-1,3-diphenyl-1,2-dihydroacridin-9(10H)-one (2e). Prepared from compound 1e (474 mg, 1.0 mmol). Yield: 330 mg, (0.77 mmol, 77%); pale yellow solid. Mp: 269–270 °C. IR νmax (film): 3060, 2899, 2803, 1619, 1540 cm−1. 1H-NMR (250 MHz, DMSO-d6) δ 12.02 (s, 1H), 8.15 (d, J = 2.3 Hz, 1H), 7.79 (dd, J = 8.8, 2.3 Hz, 1H), 7.56 (t, J = 8.9 Hz, 3H), 7.48–7.35 (m, 3H), 7.21 (t, J = 8.8 Hz, 2H), 7.13 (dd, J = 6.5, 4.7 Hz, 2H), 6.89 (d, J = 2.2 Hz, 1H), 4.61 (d, J = 7.8 Hz, 1H), 3.27 (d, J = 8.4 Hz, 1H), 3.10 (d, J = 17.4 Hz, 1H). 13C-NMR (63 MHz, DMSO-d6) δ 173.0 (CO), 146.0 (C-5), 144.2 (C-7), 138.7 (C-15), 138.2 (C-19), 134.1 (C-9), 131.6 (C-12), 129.2 (C-3), 129.0 (C-11), 128.1 (C-21), 127.2 (C-17 and C-22), 127.0 (C-20), 126.4 (C-16), 126.2 (C-18), 125.6 (C-8), 120.7 (C-10), 120.6 (C-8), 115.4 (C4), 114.2 (C14), 33.9 (C1), 33.8 (C2). Anal. Calc. for C25H18BrNO (M = 428.32): C, 70.10; H, 4.24; N, 3.27; found: C, 70.06; H, 4.28; N, 3.21.
6,8-Dichloro-1,3-diphenyl-1,2-dihydroacridin-9(10H)-one (2f). Prepared from compound 1f (464 mg, 1.0 mmol). Yield: 347 mg, (0.83 mmol, 83%); yellow solid. Mp: 199–200 °C. IR νmax (film): 3255, 3058, 2896, 1628, 1579 cm−1. 1H-NMR (250 MHz, CDCl3-d6) δ 8.38 (s, 1H), 7.46–7.32 (m, 5H), 7.26 (s, 1H), 7.22–7.05 (m, 4H), 6.49 (d, J = 2.7 Hz, 1H), 6.41 (d, J = 1.7 Hz, 1H), 6.18 (d, J = 1.8 Hz, 1H), 4.70 (d, J = 7.8 Hz, 1H), 3.30 (ddd, J = 17.3, 8.7, 2.7 Hz, 1H), 3.12 (dd, J = 17.4, 1.5 Hz, 1H). 13C-NMR (63 MHz, CDCl3-d6) δ 179.0 (CO), 152.7 (C-7), 146.4 (C-5), 143.6(C-11), 143.4(C-9), 142.2 (C-15), 139.0 (C-19), 129.0 (C-3), 128.7(C-10), 128.3 (C-17 and 22), 127.1 (C-21), 126.5(C-12), 125.7 (C-20 and C-18), 116.6 (C-4), 114.9 (C14), 110.2 (C-14, 102.0 (C-8), 101.1 (C-14), 35.0 (C1), 34.6 (C2). Anal. Calc. for C25H17Cl2NO (M = 418.31): C, 71.78; H, 4.10; N, 3.35; found: C, 71.82; H, 4.16; N, 3.31.
6,8-Dimethyl-1,3-diphenyl-1,2-dihydroacridin-9(10H)-one (2g). Prepared from compound 1g (424 mg, 1.0 mmol). Yield: 310 mg, (0.82 mmol, 82%); pale yellow solid. Mp: 275–276 °C. IR νmax (film): 3238, 3079, 2955, 2918, 1621, 1585 cm−1. 1H-NMR (250 MHz, DMSO-d6) δ 11.46 (s, 1H), 7.57–7.49 (m, 2H), 7.39 (td, J = 8.1, 2.4 Hz, 3H), 7.20 (dt, J = 5.6, 2.9 Hz, 3H), 7.17–7.06 (m, 4H), 6.84 (d, J = 2.6 Hz, 1H), 6.79 (s, 1H), 4.52 (d, J = 10 Hz), 3.31–3.16 (m, 1H), 3.02 (d, J = 17.3 Hz, 1H), 2.75 (s, 3H), 2.35 (s, J = 5.7 Hz, 3H). 13C-NMR (63 MHz, DMSO-d6) δ 176.8 (CO), 144.7 (C-7), 144.4 (C-9), 142.2 (C-5), 141.1 (C-15), 140.3 (C-12), 139.0 (C-3), 138.9 (C-19), 128.9 (C-17), 128.0 (C-21), 127.1 (C-22), 126.9 (C-16), 125.9 (C-20), 125.4 (C-18), 121.4 (C-10), 117.1 (C-12), 115.5 (C-4), 114.6 (C-14), 34.4 (C-1), 33.9 (C-2), 23.2 (Me), 21.1 (Me). Anal. Calc. for C27H23NO (M = 377.48): C, 85.91; H, 6.14; N, 3.71; found: C, 85.86; H, 6.16; N, 3.67.
3-(4-Bromophenyl)-1-phenyl-1,2-dihydroacridin-9(10H)-one (2i). Prepared from compound 1i (474 mg, 1.0 mmol). Yield: 407 mg, (0.95 mmol, 95%); yellow solid. Mp: 317–318 °C. IR νmax (film): 2774, 1631, 1578, 1487 cm−1. 1H-NMR (250 MHz, DMSO-d6) δ 11.85 (s, 1H), 8.06 (d, J = 7.3 Hz, 1H), 7.68–7.59 (m, 3H), 7.53 (t, J = 8.2 Hz, 3H), 7.36–7.05 (m, 6H), 6.91 (d, J = 2.5 Hz, 1H), 4.60 (d, J = 7.9 Hz, 1H), 3.31–3.20 (m, 1H), 3.03 (d, J = 16.8 Hz, 1H). 13C-NMR (126 MHz, DMSO-d6) δ 174.1 (CO), 144.2 (C-5), 143.8 (C-15), 143.0 (C-7), 139.1 (C-3), 137.9 (C-19), 131.6 (C-21), 131.0 (C-9), 127.7 (C-17), 127.3 (C-16), 126.7 (C-20), 125.7 (C-12), 124.9 (C-18), 124.8 (C-11), 122.4 (C-10), 121.9 (C-22), 117.7 (C-8), 117.7 (C4), 113.7 (C14), 33.6 (C1), 33.6 (C2). Anal. Calc. for C25H18BrNO (M = 428.32): C, 70.10; H, 4.24; N, 3.27; found: C, 70.07; H, 4.26; N, 3.23.
1-Phenyl-3-(thiophen-2-yl)-1,2-dihydroacridin-9(10H)-one (2j). Prepared from compound 1j (402 mg, 1.0 mmol). Yield: 334 mg, (0.94 mmol, 94%); orange solid. Mp: 304–305 °C. IR νmax (film): 3068, 2923, 1619, 1572 cm−1. 1H-NMR (250 MHz, DMSO-d6) δ 11.82 (s, 1H), 8.06 (d, J = 8.0 Hz, 1H), 7.64 (dd, J = 11.2, 5.9 Hz, 2H), 7.53 (d, J = 8.2 Hz, 1H), 7.46 (d, J = 3.4 Hz, 1H), 7.35–7.06 (m, 7H), 6.86 (s, 1H), 4.61 (br s, 1H), 3.21 (br s, 2H). 13C-NMR (63 MHz, DMSO-d6) δ 174.4 (CO), 162.7 (C-5), 144.7 (C-15), 143.7(C-3), 143.1 (C-7), 139.6 (C-19), 139.5 (C-9), 131.7 (C-22), 128.9 (C-17), 128.4 (C-21), 128.4 (C-12), 127.4 (C-20), 127.1 (C-16), 126.4 (C-11), 125.4 (C-18), 123.1 (C-10), 118.3 (C-4), 115.0 (C-8), 114.1 (C14), 34.1 (C1), 34.0 (C2). Anal. Calc. for C23H17NOS (M = 355.45): C, 77.72; H, 4.82; N, 3.94; found: C, 77.67; H, 4.84; N, 3.91.
1,3-Di(furan-2-yl)-1,2-dihydroacridin-9(10H)-one (2k). Prepared from compound 1k (375 mg, 1.0 mmol). Yield: 303 mg (0.92 mmol, 92%); pale yellow solid. Mp: 282–283 °C. IR νmax (film): 3066, 2911, 1621, 1562 cm−1. 1H-NMR (250 MHz, DMSO-d6) δ 11.84 (s, 1H), 8.08 (d, J = 8.1 Hz, 1H), 7.84 (d, J = 1.6 Hz, 1H), 7.63 (t, J = 7.6 Hz, 1H), 7.52 (d, J = 7.8 Hz, 1H), 7.43 (s, J = 0.9 Hz, 1H), 7.29 (t, J = 8.0 Hz, 1H), 6.95 (d, J = 3.4 Hz, 1H), 6.84 (d, J = 2.3 Hz, 1H), 6.62 (dd, J = 3.4, 1.8 Hz, 1H), 6.19 (dd, J = 3.1, 1.8 Hz, 1H), 5.76 (d, J = 3.2 Hz, 1H), 4.62 (d, J = 7.4 Hz, 1H), 3.16 (dd, J = 17.1, 1.1 Hz, 1H), 2.91 (ddd, J = 17.1, 8.0, 2.5 Hz, 1H). 13C-NMR (63 MHz, DMSO-d6) δ 173.8 (CO), 156.3 (C-15), 152.2 (C-5), 145.1 (C-19), 143.5 (C-22), 141.5 (C-18), 139.3 (C-7), 134.1 (C-3), 131.4 (C-9), 125.1 (C-12), 125.0 (C-11), 122.8 (C-10), 118.0 (C4), 112.5(C-8), 112.5 (C-21), 111.4 (C-20), 111.4 (C-14), 110.2 (C-17), 105.1 (C-16), 28.7 (C1), 28.2 (C2). Anal. Calc. for C21H15NO3 (M = 329.35): C, 76.58; H, 4.59; N, 4.25; found: C, 76.53; H, 4.62; N, 4.28.
3-(4-Methoxyphenyl)-1-phenyl-1,2-dihydroacridin-9(10H)-one (2l). Prepared from compound 1l (200 mg, 0.47 mmol); yield: 76 mg (0.20 mmol, 43% yield). yellow solid Mp: 242 °C. IR νmax (film): 3391, 2990, 2770, 2106, 1607. 1H-NMR (250 MHz, DMSO-d6) δ 11.87 (s, 1H), 8.08 (dd, J = 8.1, 1.4 Hz, 1H), 7.68–7.53 (m, 4H), 7.48–7.37 (m, 3H), 7.29 (ddd, J = 8.1, 6.7, 1.3 Hz, 1H), 7.17–7.08 (m, 2H), 6.90 (d, J = 2.5 Hz, 1H), 6.78–6.65 (m, 2H), 4.57 (d, J = 7.8 Hz, 1H), 3.63 (s, 3H), 3.32–3.19 (ddd, J = 17.0, 8.3, 2.5 Hz, 1H), 3.10–3.01 (d, J = 17.0 Hz, 1H). 13C-NMR (63 MHz, DMSO-d6) δ 174.7 (CO), 157.9(C-22), 145.7 (C-5), 143.8 (C-15), 139.6 (C-7), 139.1 (C-3), 136.7 (C-9), 131.7 (C-19), 129.4 (C-20), 129.3 (C-17), 128.3 (C-16 and C-18), 125.8 C-12), 125.4 (C-11), 123.1 (C-10), 118.3 (C-8), 117.4 (C4), 114.5 (C-21), 113.7 (C14), 55.2 (OMe), 34.4 (C1), 33.3 (C2). Anal. Calc. for C26H21O2N C, 82.30; H, 5.58; N, 3.69. Found C, 80.91; H, 5.47; N, 3.85.
3-(4-Chlorophenyl)-1-phenyl-1,2-dihydroacridin-9(10H)-one (2m). Prepared from compound 1m (300 mg, 0.7 mmol); yield: 140 mg (0.4 mmol, 52% yield). Brown solid Mp: 278 °C. IR νmax (film): 3252, 3056, 2874, 2765, 2107, 1700, 1620. 1H-NMR (250 MHz, DMSO-d6) δ 11.90 (s, 1H), 8.06 (dd, J = 8.1, 1.4 Hz, 1H), 7.69–7.51 (m, 5H), 7.50–7.37 (m, 3H), 7.29 (m, 2H), 7.20 (m, 2H), 6.90 (d, J = 2.5 Hz, 1H), 4.59 (d, J = 8.1 Hz, 1H), 3.24 (dd, J = 8.5, 2.3 Hz, 1H), 3.07 (dd, J = 17.6, 1.4 Hz, 1H). 13C-NMR (63 MHz, DMSO-d6) δ 174.6 (CO), 145.6 (C-5), 144.0 (C-15), 143.8 (C-7), 139.6 (C-3), 139.0 (C-9), 131.8(C-19), 131.0 C-22), 129.5 (C-21), 129.3 (C-17), 129.3 (C-20 y C-16), 128.3 (C-11), 125.9 (C-12), 125.4 (C-18), 123.2 (C-10), 118.4 (C-8), 117.5 (C4), 113.6 (C14), 34.0 (C1), 33.6 (C2). Anal. Calc. for C25H18ONCl C, 78.22; H, 4.73; N, 3.65. Found C, 76.03; H, 4.85; N, 3.97.
3-(2,4-Dimethoxyphenyl)-1-(4-methoxyphenyl)-1,2-dihydroacridin-9(10H)-one (2n). Prepared from compound 1n (150 mg, 0.3 mmol); 58 mg (0.13 mmol, 44% yield). Yellow solid Mp: 168 °C. IR νmax (film): 3214, 3066, 2932, 2829, 2118, 1603. 1H-NMR (250 MHz, DMSO-d6) δ 12.14 (s, 1H), 8.15–8.07 (m, 1H), 7.65 (dd, J = 6.2, 1.5 Hz, 2H), 7.37–7.28 (m, 1H), 7.24 (dd, J = 8.5, 1.8 Hz, 1H), 7.20–7.11 (m, 2H), 6.86 (d, J = 2.4 Hz, 1H), 6.75 (dd, J = 8.9, 2.5 Hz, 2H), 6.66–6.60 (m, 1H), 6.57 (d, J = 2.4 Hz, 1H), 4.52 (d, J = 7.7 Hz, 1H), 3.81 (s, 3H), 3.79 (s, 3H), 3.66 (s, 3H), 3.28–3.17 (m, 1H), 2.92 (d, J = 17.3 Hz, 1H). 13C-NMR (63 MHz, DMSO-d6) δ 161.4 (CO), 158.7 (C-22), 157.9 (C-18 and C-20), 145.6 (C-5), 144.7 (C-7), 139.5 (C-3), 136.6 (C-15), 131.7 (C-9), 129.6 (C-16), 128.4 (C-12), 125.2 (C-20), 125.0(C-11), 121.8 (C-8), 118.6 (C-17),118.4 (C4), 114.0 (C-21), 113.6 (C14), 105.6 (C-19), 99.3 (C-21′), 56.0 (OMe), 55.7 (OMe), 55.3 (OMe), 36.3 (C2), 33.5 (C1). Anal. Calc. for C28H25O4N C, 76.52; H, 5.73; N, 3.19. Found C, 75.31; H, 5.67; N, 3.38.
1,3-Bis(4-chlorophenyl)-1,2-dihydroacridin-9(10H)-one (2o). Prepared from compound 1o (173 mg, 0.3 mmol); 34 mg (0.08 mmol, 27% yield). Yellow solid Mp: 287 °C. IR νmax (film): 3252, 3059, 2749, 2681, 2113, 1624. 1H-NMR (300 MHz, DMSO-d6) δ 11.86 (s, 1H), 8.07 (dd, J = 8.1, 1.5 Hz, 1H), 7.68–7.56 (m, 3H), 7.53–7.47 (m, 2H), 7.30 (ddd, J = 8.1, 6.8, 1.1 Hz, 2H), 7.23 (s, 4H), 6.91 (d, J = 2.7 Hz, 1H), 4.60 (d, J = 8.3 Hz, 1H), 3.29–3.22 (m, 1H), 3.04 (dd, J = 17.6, 1.5 Hz, 1H). 13C-NMR (75 MHz, DMSO-d6) δ 174.7 (CO), 144.3 (C-5), 143.8 (C-15), 139.7 (C-7), 138.0 (C-3), 134.1 (C-19), 132.0 (C-18), 131.1 (C-9), 129.4 (C-22), 129.3 (C-16), 128.5 (C-17), 127.8 (C-21), 125.5 (C-20), 125.4 (C-12), 124.5 (C-11), 123.4 (C-10), 118.5 (C-8), 118.3 (C4), 113.8 (C14), 34.0 (C1), 33.7 (C2). Anal. Calc. for C25H17ONCl2 C, 71.78; H, 4.10; N, 3.35. Found C, 71.04; H, 4.20; N, 3.49.
3.4. General Procedure for the Synthesis of 1,3-diaryl-acridin-9(10H)-ones 3
A microwave tube containing a solution of the suiTable 1,3-diaryl-1,2-dihydroacridin-9(10H)-one derivatives 2 (30 to 441 mg, 0.07 to 1.0 mmol) in nitrobenzene (3 mL), was closed and placed in the cavity of a CEM Discover focused microwave oven. The reaction mixture was heated by microwave irradiation for 90 min., at 200 W and 250 °C. Then, the mixture was cooled to room temperature and the solvent was evaporated under reduce pressure. The crude mixture was washed with cool chloroform and the solid obtained was filtered to obtain compounds 3. In the cases of compounds 3k and 3n, purification required column chromatography on silica gel, eluting with petrol ether/EtOAc (7/3). Compound numbering used in the assignment of 13C-NMR signals is given below.
![Molecules 25 05565 i012]()
1,3-Diphenylacridin-9(10H)-one (3a). Prepared from compound 2a (175 mg, 0.5 mmol). Yield: 142 mg, (0.41 mmol, 82%); brown solid. Mp: 333–334 °C. IR νmax (film): 3062, 2972, 1624, 1594 cm−1. 1H-NMR (250 MHz, DMSO-d6) δ 8.03 (d, J = 8.1 Hz, 1H), 7.79 (d, J = 8.3 Hz, 3H), 7.71 (t, J = 6.9 Hz, 1H), 7.58–7.45 (m, 4H), 7.33 (bs, 5H), 7.24–7.14 (m, 2H). 13C-NMR (63 MHz, DMSO-d6) δ 176.5 (CO), 144.1 (C-5), 143.5 (C-3), 143.1 (C-7), 142.9 (C-19), 140.4 (C-15), 138.8 (C-1), 133.3 (C-9), 129.3 (C-21), 128.7 (C_17), 127.1 (C-20, C-16 and C-22) (3 overlapped signals), 126.3 (C-18), 126.2 (C-11), 123.2 (C-14), 121.9 (C-10), 121.0 (C-12), 116.8 (C-8), 116.6 (C-4), 114.5 (C-2). Anal. Calc. for C25H17NO (M = 347.41): C, 86.43; H, 4.93; N, 4.03; found: C, 85.94; H, 5.03; N, 4.06.
7-(Dimethylamino)-1,3-diphenylacridin-9(10H)-one (3b). Prepared from compound 2b (196 mg, 0.5 mmol). Yield: 156 mg, (0.40 mmol, 80%); brown oil. IR νmax (film): 2924, 2853, 1621, 1589 cm−1. 1H-NMR (250 MHz, CDCl3) δ 8.82 (s, 1H), 7.64 (dd, J = 7.9, 1.5 Hz, 2H), 7.50–7.39 (m, 8H), 7.39–7.32 (m, 2H), 7.24 (d, J = 1.7 Hz, 1H), 7.16 (d, J = 8.8 Hz, 1H), 7.01 (dd, J = 8.8, 2.7 Hz, 1H), 2.79 (s, 6H). 13C-NMR (63 MHz, CDCl3) δ 177.7 (CO), 144.9 (C-5), 144.6 (C-3), 144.0 (C-10), 143.8 (C-19), 141.8 (C-15), 139.6 (C-1), 133.2 (C-7), 129.1 (C-21), 128.5 (C-17), 128.4 (C-16), 127.5 (C-20), 127.4 (C-18), 126.6 (C-22), 124.0 (C-14), 123.9 (C_12), 122.8 (C-9), 117.5 (C-4), 116.7 (C-2), 114.3 (C-11), 105.2 (C-8), 31.3 (NMe). Anal. Calc. for C27H22N2O (M = 390.48): C, 83.05; H, 5.68; N, 7.17; found: C, 82.95; H, 6.03; N, 7.15.
7-Fluoro-1,3-diphenylacridin-9(10H)-one (3c). Prepared from compound 2c (184 mg, 0.5 mmol). Yield: 155 mg, (0.42 mmol, 85%); pale yellow solid. Mp: 320–321 °C. IR νmax (film): 3238, 3100, 2969, 1625, 1594, 1563 cm−1. 1H-NMR (250 MHz, DMSO-d6) δ 11.93 (bs, 1H), 7.84–7.73 (m, 3H), 7.69 (d, J = 8.0 Hz, 1H), 7.66–7.59 (m, 2H), 7.59–7.45 (m, 3H), 7.35 (bs, 5H), 7.19 (d, J = 0.8 Hz, 1H). 13C-NMR (63 MHz, DMSO-d6) δ 175.7 (CO), 157.0 (C_10) (d, J = 240 Hz), 143.9 (C-5), 143.6 (C_3), 142.9 (C-19), 142.8 (C-15), 138.7 (C-1), 137.2 (C-7), 129.3 C-17), 128.7 (C-21), 128.7 (C-16), 127.2 (C-20), 126.3 (C-18), 123.4 (C-22), 122.5 (C-14), 122.4 (C-12), 122.1 (C-9) (d, J = 25.2 Hz), 119.4 (C-8), 115.7 (C-2), 114.5 (C-4), 110.1 (d, J = 22.7 Hz) (C-11). Anal. Calc. for C25H16FNO (M = 365.40): C, 82.18; H, 4.41; N, 3.83; found: C, 82.13; H, 4.47; N, 3.81.
7-Chloro-1,3-diphenylacridin-9(10H)-one (3d). Prepared from compound 2d (192 mg, 0.5 mmol). Yield: 145 mg, (0.38 mmol, 76%); yellow solid. Mp: 312–313 °C. IR νmax (film): 3068, 2967, 1626, 1561 cm−1. 1H-NMR (250 MHz, DMSO-d6) δ 11.97 (s, 1H), 7.96 (d, J = 2.5 Hz, 1H), 7.81 (s, 1H), 7.79–7.74 (m, 3H), 7.72 (d, J = 2.5 Hz, 1H), 7.56 (dd, J = 7.9, 4.1 Hz, 3H), 7.49 (dd, J = 8.0, 3.7 Hz, 2H), 7.35 (s, 3H), 7.20 (d, J = 1.7 Hz, 1H). 13C-NMR (63 MHz, DMSO-d6) δ 175.4 (CO), 144.1 (C-5), 143.8 (C-3), 142.8 (C-19), 139.0 (C-15), 138.7 (C-7), 133.7 (C-1), 133.3 (C-9), 129.3 (C-11), 128.8 (C-17), 128.7 (C-21), 127.2 (C-16), 127.2 (C-20), 126.4 (C-18), 125.4 (C-22), 125.1 (C-14), 123.7 (C-12), 122.7 (C-8), 119.3 (C-10), 116.4 (C-4), 114.6 (C-2). Anal. Calc. for C25H16ClNO (M = 381.85): C, 78.63; H, 4.22; N, 3.67; found: C, 78.58; H, 4.26; N, 3.63.
7-Bromo-1,3-diphenylacridin-9(10H)-one (3e). Prepared from compound 2e (214 mg, 0.5 mmol). Yield: 166 mg, (0.69 mmol, 78%); yellow solid. Mp: 276–277 °C. IR νmax (film): 3264, 3059, 2985, 1620, 1596 cm−1. 1H-NMR (250 MHz, DMSO-d6) δ 11.99 (s, 1H), 8.10 (d, J = 2.3 Hz, 1H), 7.83–7.79 (m, 2H), 7.76 (m, 3H), 7.58–7.52 (m, 2H), 7.52–7.46 (m, 3H), 7.38–7.33 (m, 3H), 7.20 (d, J = 1.6 Hz, 1H). 13C-NMR (63 MHz, DMSO-d6) δ 175.3 (CO), 144.1 (C-5), 143.8 (C-3), 142.8 (C-19), 139.3 (C-7), 138.7 (C-15), 135.8 (C-1), 129.3 (C-9), 128.8 (C-11), 128.7 (C-17), 128.3 (C-21), 127.2 (C-16), 127.2 (C-20), 127.1 (C-18), 126.4 (C-229, 123.7 (C-14), 123.2 (C-12), 119.5 (C-8), 116.5 (C-10), 114.6 (C-4), 113.1 (C-2). Anal. Calc. for C25H16BrNO (M = 426.30): C, 70.43; H, 3.78; N, 3.29; found: C, 70.39; H, 3.73; N, 3.34.
6,8-Dichloro-1,3-diphenylacridin-9(10H)-one (3f). Prepared from compound 2f (209 mg, 0.5 mmol). Yield: 162 mg, (0.39 mmol, 78%); yellow solid. Mp: 281–282 °C. IR νmax (film): 3279, 3060, 2923, 1620, 1595 cm−1. 1H-NMR (250 MHz, DMSO-d6) δ 11.61 (s, 1H), 9.68 (d, J = 5.0 Hz, 1H), 7.75 (d, J = 7.2 Hz, 2H), 7.62 (s, 1H), 7.57–7.43 (m, 3H), 7.39–7.22 (m, 4H), 7.09 (d, J = 0.8 Hz, 1H), 6.51 (d, J = 1.0 Hz, 1H), 6.10 (d, J = 1.0 Hz, 1H). 13C-NMR (63 MHz, DMSO-d6) δ 179.2 (CO), 153.3 (C-7), 143.7 (C-5), 143.5 (C-3), 143.4 (C-19), 143.4 (C-15), 141.9 (C-11), 139.3 (C-1), 138.7 (C-9), 129.2 (C-17), 128.7 (C-21), 128.4 (C-16), 127.2 (C-20), 127.1 (C-18), 126.2 (C-22), 123.8 (C-12), 117.4 (C-14), 113.7 (C-10), 106.0 (C-8), 99.9 (C-4), 98.9 (C-2). Anal. Calc. for C25H15Cl2NO (M = 416.30): C, 72.13; H, 3.63; N, 3.36; found: C, 72.09; H, 3.67; N, 3.31.
6,8-Dimethyl-1,3-diphenylacridin-9(10H)-one (3g). Prepared from compound 2g (189 mg, 0.5 mmol). Yield: 150 mg, (0.40 mmol, 80%); brown solid. Mp: 286–287 °C. IR νmax (film): 3021, 2962, 1593, 1534 cm−1. 1H-NMR (250 MHz, DMSO-d6) δ 11.42 (s, 1H), 7.76 (d, J = 7.2 Hz, 2H), 7.66 (s, 1H), 7.58–7.42 (m, 3H), 7.34 (m, 5H), 7.10 (s, 2H), 6.75 (s, 1H), 2.60 (s, 3H), 2.37 (s, 3H). 13C-NMR (63 MHz, DMSO-d6) δ 178.7 (CO), 143.8 (C-5), 143.3 (C-7), 143.0 (C-9), 142.3 (C-3), 142.1 (C-19), 142.1 (C-11), 139.9 (C-15), 139.0 (C-1), 129.2 (C.12), 128.5 (C-17), 128.5 (C-21), 127.3 (C-16), 127.1 (C-20), 126.1 (C-18), 125.4 (C-22), 123.0 (C-14), 118.6 (C-10), 118.2 (C-4), 114.3 (C-2), 113.7 (C-8), 23.2 (C9-Me), 21.3 (C11-Me). Anal. Calc. for C27H21NO (M = 375.46): C, 86.37; H, 5.64; N, 3.73; found: C, 86.32; H, 5.68; N, 3.72.
1-(4-Nitrophenyl)-3-phenylacridin-9(10H)-one (3h). Prepared from compound 1h (441 mg, 1.0 mmol). Yield: 338 mg, (0.86 mmol, 86 % overall) without the need for a separate oxidation step; pink solid. Mp: 174–175 °C. IR νmax (film): 3401, 3325, 3260, 1587, 1493 cm−1. 1H-NMR (250 MHz, DMSO-d6) δ 8.30 (s, 1H), 7.66 (d, J = 7.0 Hz, 2H), 7.46 (t, J = 7.3 Hz, 1H), 7.38 (d, J = 8.6 Hz, 3H), 7.31–7.23 (m, 1H), 7.22–7.12 (m, 5H), 6.84 (t, J = 7.2 Hz, 1H), 6.65 (d, J = 8.5 Hz, 2H). 13C-NMR (63 MHz, DMSO-d6) δ 182.3 (CO), 148.6 (C-18), 144.3 (C-15), 143.4 (C-5), 142.5 (C-3), 141.8 (C-7), 140.9 (C-19), 129.3 (C21), 128.9 (C-1), 127.7 (C-9), 127.5 (C-16), 127.4 (C-20 and 22), 126.8 (C-11 and C14), 119.9 (C-17), 117.1 (C-10), 116.0 (C-12), 114.2 (C-8), 112.9 (C-4), 112.5 (C_2). Anal. Calc. for C25H16N2O3 (M = 392.41): C, 76.52; H, 4.11; N, 7.14; found: C, 76.48; H, 4.05; N, 7.18.
3-(4-Bromophenyl)-1-phenylacridin-9(10H)-one (3i). Prepared from compound 2i (214 mg, 0.5 mmol). Yield: 179 mg, (0.42 mmol, 84%); yellow solid. Mp: 326–327 °C. IR νmax (film): 3090, 2984, 1624, 1570, 1533 cm−1. 1H-NMR (250 MHz, DMSO-d6) δ 11.80 (s, 1H), 8.03 (d, J = 7.1 Hz, 1H), 7.81–7.63 (m, 6H), 7.52 (d, J = 8.2 Hz, 1H), 7.41–7.29 (m, 5H), 7.20 (t, J = 7.7 Hz, 1H), 7.16 (d, J = 1.6 Hz, 1H). 13C-NMR (63 MHz, DMSO-d6) δ 176.8 (CO), 144.5 (C-5), 143.3 (C-3), 143.2 (C-7), 142.5 (C-15), 140.7 (C-19), 138.3 (C-1), 133.7 (C-9), 132.5 C-20), 129.6 (C-17), 129.0 (C-16), 127.5 (C-18), 126.6 (C-11), 126.5 (C-14), 123.3 (C-22), 122.6 (C-21), 122.3 (C-10), 121.4 (C-12), 117.2 (C-8), 117.1 (C-4), 114.8 (C-2). Anal. Calc. for C25H16BrNO (M = 426.30): C, 70.43; H, 3.78; N, 3.29; found: C, 70.39; H, 3.82; N, 3.34.
1-Phenyl-3-(thiophen-2-yl)acridin-9(10H)-one (3j). Prepared from compound 2j (178 mg, 0.5 mmol). Yield: 140 mg, (0.39 mmol, 79%); orange solid. Mp: 301–302 °C IR νmax (film): 3057, 3007, 2922, 1673, 1592 cm−1. 1H-NMR (250 MHz, DMSO-d6) δ 11.76 (s, 1H), 8.01 (dd, J = 8.1, 1.1 Hz, 1H), 7.78–7.64 (m, 4H), 7.49 (d, J = 8.1 Hz, 1H), 7.40–7.27 (m, 5H), 7.25–7.14 (m, 3H). 13C-NMR (63 MHz, DMSO-d6) δ 176.2 (CO), 144.3 (C-5), 142.9 (C-7), 142.9 (C-19), 141.7 (C-15), 140.4 (C-3), 136.8 (C-1), 133.3 (C-9), 129.0 (C-17), 128.5 (C-22), 127.8 (C-21), 127.2 (C-16), 126.3 (C-20), 126.2 (C-18), 125.9 (C-14), 122.0 (C-11), 121.5 (C-10), 121.1 (C-12), 116.8 (C-8), 116.6 (C-4), 112.5 (C-2). Anal. Calc. for C23H15NOS (M = 353.44): C, 78.16; H, 4.28; N, 3.96; found: C, 78.12; H, 4.24; N, 4.04.
1,3-Di(furan-2-yl)acridin-9(10H)-one (3k). Prepared from compound 2k (165 mg, 0.5 mmol). Yield: 126 mg, (0.38 mmol, 77%); brown solid. Mp: 195–196 °C. IR νmax (film): 3104, 2922, 1621, 1601 cm−1. 1H-NMR (250 MHz, CDCl3) δ 10.10 (s, 1H), 8.33 (d, J = 8.0 Hz, 1H), 7.63 (d, J = 1.4 Hz, 1H), 7.56–7.45 (m, 3H), 7.39 (dd, J = 8.0, 1.4 Hz, 2H), 7.11 (t, J = 7.5 Hz, 1H), 6.69 (d, J = 3.3 Hz, 1H), 6.57 (d, J = 3.0 Hz, 1H), 6.47–6.38 (m, 2H). 13C-NMR (63 MHz, CDCl3) δ 177.7 (CO), 154.5 (C-15), 152.2 (C-19), 143.6 (C-5), 142.7 (C-18), 142.2 (C-22), 140.2 (C-7), 133.9 (C-3), 133.3 (C-9), 132.5 (C-1), 127.1 (C-11), 122.7 (C-14), 121.8 (C-10), 121.1 (C-12), 117.6 (C-8), 116.7 (C-4), 112.3 (C-2), 112.1 (C-17), 111.1 (C-21), 108.4 (C-16), 108.2 (C-20). Anal. Calc. for C21H13NO3 (M= 327.33): C, 77.05; H, 4.00; N, 4.28; found: C, 77.11; H, 4.05; N, 4.32.
3-(4-Methoxyphenyl)-1-phenylacridin-9(10H)-one (3l). Prepared from compound 2l (50 mg, 0.13 mmol); Yield: 38 mg (0.10 mmol, 74% yield); yellow solid Mp: 296 °C. IR νmax (film): 3264, 3107, 2929, 2106, 1889, 1617..1H-NMR (300 MHz, CDCl3) δ 9.18 (s, 1H), 8.36 (dd, J = 8.2, 1.5 Hz, 1H), 7.69–7.59 (m, 3H), 7.55 (d, J = 1.8 Hz, 1H), 7.50–7.41 (m, 4H), 7.41–7.33 (m, 3H), 7.22 (ddd, J = 8.1, 7.0, 1.0 Hz, 1H), 6.99–6.92 (m, 2H), 3.83 (s, 3H). 13C-NMR (75 MHz, CDCl3) δ 177.1 (CO), 158.8 (C-22), 145.1 (C-5), 144.9 (C-3), 144.1 (C-7), 140.2 (C-15), 139.2 (C-1), 134.8 (C-19), 133.5 (C-9), 129.7 (C-20), 129.0 (C-17), 128.6 (C-16), 127.4 (C-18), 127.1 (C-11), 125.0 (C-14), 122.1 (C-10), 122.0 (C-12), 117.0 (C-21), 116.4 (C-8), 114.2 (C-4), 113.2(C-2), 55.2 (OMe). Anal. Calc. for C26H19O2N C, 82.74; H, 5.07; N, 3.71. Found C, 79.83; H, 5.18; N, 3.69.
3-(4-Chlorophenyl)-1-phenylacridin-9(10H)-one (3m). Prepared from compound 2m (30 mg, 0.08 mmol); Yield: 22 mg (0.06 mmol, 70% yield). Yellow solid Mp: 366 °C. IR νmax (film):3262, 3072, 2930, 2109, 1892, 1623. 1H-NMR (300 MHz, DMSO-d6) δ 11.87 (s, 1H), 8.10 (dd, J = 8.1, 1.5 Hz, 1H), 7.86 (dd, J = 7.2, 1.8 Hz, 3H), 7.78 (ddd, J = 8.5, 6.9, 1.6 Hz, 1H), 7.61 (t, J = 7.2 Hz, 3H), 7.56–7.51 (m, 1H), 7.51–7.39 (m, 4H), 7.31–7.23 (m, 2H). 13C-NMR (75 MHz, DMSO-d6) δ 176.9 (CO), 144.0 (C-5), 143.3 (C-3), 143.1 (C-7), 142.4 (C-15), 140.9 (C-19), 139.2 (C-1), 133.8 (C-9), 131.6 (C-22), 130.9 (C-21), 129.7 (C-20), 129.2 (C-17), 127.6 (C-16), 127.5 (C-18), 126.6 (C-11), 123.5 (C-14), 122.3 (C-10), 121.6 (C-12), 117.3 (C-6), 116.9 (C.4), 115.2 (C-2). Anal. Calc. for C25H16ONCl C, 78.64; H, 4.22; N, 3.67. Found C, 76.90; H, 4.26; N, 3.67.
3-(2,4-Dimethoxyphenyl)-1-(4-methoxyphenyl)-acridin-9(10H)-one (3n). Prepared from compound 2n (30 mg, 0.07 mmol); Yield: 24 mg (0.05 mmol, 77% yield). Orange solid Mp: 281 °C. IR νmax (film): 3263, 3105, 2954, 2831, 2110, 1887, 1611. 1H-NMR (300 MHz, CDCl3) δ 9.37 (s, 1H), 8.24 (dd, J = 8.2, 1.5 Hz, 1H), 7.57 (d, J = 1.7 Hz, 1H), 7.52 (ddd, J = 8.4, 6.9, 1.5 Hz, 1H), 7.36 (d, J = 8.3 Hz, 1H), 7.31–7.22 (m, 3H), 7.16–7.08 (m, 2H), 6.93–6.84 (m, 2H), 6.51–6.44 (m, 2H), 3.78 (s, 3H), 3.77 (s, 3H), 3.74 (s, 3H). 13C-NMR (75 MHz, CDCl3) δ 176.8 (CO), 161.3 (C-20), 158.8 (C-22), 157.8 (C-18), 142.9 (C-5), 142.6 (C-3), 141.9 (C-7), 140.0 (C-1), 134.9 (C-9), 133.3 (C-15), 131.5 (C-16), 129.9 (C-20), 127.5 (C-11), 127.0 (C-14 and C-19), 121.9 (C-10), 121.8 (C-12), 121.4 (C-17), 116.7 (C-8), 116.3 (C-4), 113.2 (C-2), 105.0 (C-21), 99.1 (C21′), 55.7 (OMe), 55.5 (OMe), 55.2 (OMe). Anal. Calc. for C28H23O4N C, 76.87; H, 5.30; N, 3.20. Found C, 74.15; H, 5.41; N, 3.15.
1,3-Bis(4-chlorophenyl)acridin-9(10H)-one (3o). Prepared from compound 2o (30 mg, 0.07 mol); Yield: 13 mg (0.03 mmol, 43% yield). Orange solid Mp 298 °C. IR νmax (film): 3264, 3114, 2922, 2105, 1619. 1H-NMR (300 MHz, DMSO-d6) δ 11.84 (s, 1H), 8.04 (dd, J = 8.2, 1.6 Hz, 1H), 7.87–7.81 (m, 2H), 7.78 (d, J = 1.8 Hz, 1H), 7.72 (ddd, J = 8.5, 6.9, 1.6 Hz, 1H), 7.63–7.58 (m, 2H), 7.54 (d, J = 8.3 Hz, 1H), 7.45–7.34 (m, 4H), 7.26–7.18 (m, 2H). 13C-NMR (75 MHz, DMSO-d6) δ 176.8 (CO), 143.3 (C-5), 142.7 (C-3), 142.4 (C-7), 140.9 (C-19), 137.9 (C-15), 134.1 (C-1), 133.9 (C-9), 131.7 (C-18), 131.6 (C-22), 130.9 (C-17), 130.1 (C-21), 129.7 (C-16), 129.4 (C-20), 127.5 (C-11), 126.7 (C-14), 123.3 (C-10), 122.4 (C-12), 117.4 (C-8), 117.0 (C-4), 115.3 (C-2). Anal. Calc. for C25H15ONCl2 C, 72.13; H, 3.63; N, 3.36. Found C, 71.23; H, 3.76; N, 3.53.