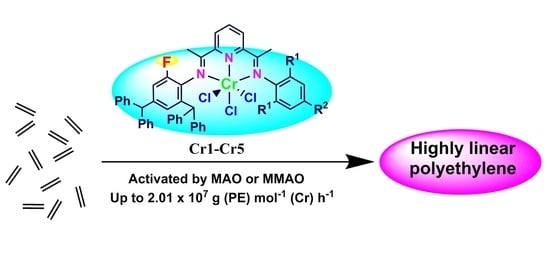
Unifying Molecular Weights of Highly Linear Polyethylene Waxes through Unsymmetrical 2,4-Bis(imino)pyridylchromium Chlorides
Abstract
:1. Introduction
2. Results
2.1. Synthesis and Characterization
2.2. X-ray Crystallographic Studies
2.3. Ethylene Polymerization
2.3.1. Catalytic Evaluation of Cr4/MAO Catalytic System
2.3.2. Ethylene Polymerization with the Cr1–Cr5/MAO Using Optimal Reaction Conditions
2.3.3. Catalytic Evaluation of Cr4/MMAO Catalytic System
2.3.4. Ethylene Polymerization with the Cr1–Cr5/MMAO Using Optimal Reaction Conditions
3. Materials and Methods
3.1. General Considerations
3.2. Synthesis of 2-Acetyl-6-(1-(2,4-dibenzhydryl-6-fluorophenylimino)ethyl)pyridine (1) and Ligands L1–L5
3.2.1. Synthesis of 2-Acetyl-6-(1-(2,4-dibenzhydryl-6-fluorophenylimino)ethyl)pyridine (1)
3.2.2. Synthesis of 2-(1-(2,4-Dibenzhydryl-6-fluorophenylimino)ethyl)-6-(1-(2,6-dimethylphenyl-imino)ethyl)pyridine (L1)
3.2.3. Synthesis of 2-(1-(2,4-Dibenzhydryl-6-fluorophenylimino)ethyl)-6-(1-(2,6-diethylphenylimino) ethyl)pyridine (L2)
3.2.4. Synthesis of 2-(1-(2,4-Dibenzhydryl-6-fluorophenylimino)ethyl)-6-(1-(2,6-diisopropylphenyl imino)ethyl)pyridine (L3)
3.2.5. Synthesis of 2-(1-(2,4-Dibenzhydryl-6-fluorophenylimino)ethyl)-6-(1-(mesitylimino)ethyl) pyridine (L4)
3.2.6. Synthesis of 2-(1-(2,4-Dibenzhydryl-6-fluorophenylimino)ethyl)-6-(1-(2,6-diethyl-4-methyl- phenyl imino)ethyl)pyridine (L5)
3.3. Synthesis of Chromium Complexes Cr1–Cr5
3.3.1. Synthesis of 2-(1-(2,4-Dibenzhydryl-6-fluorophenylimino)ethyl)-6-(1-(2,6-dimethylphenyl imino)ethyl)pyridylchromium(III) chloride (Cr1)
3.3.2. Synthesis of 2-(1-(2,4-Dibenzhydryl-6-fluorophenylimino)ethyl)-6-(1-(2,6-dimethylphenyl imino)ethyl)pyridylchromium(III) chloride (Cr2)
3.3.3. Synthesis of 2-(1-(2,4-Dibenzhydryl-6-fluorophenylimino)ethyl)-6-(1-(2,6-dimethylphenyl imino)ethyl)pyridylchromium(III) chloride (Cr3)
3.3.4. Synthesis of 2-(1-(2,4-Dibenzhydryl-6-fluorophenylimino)ethyl)-6-(1-(2,6-dimethylphenyl imino)ethyl)pyridylchromium(III) chloride (Cr4)
3.3.5. Synthesis of 2-(1-(2,4-Dibenzhydryl-6-fluorophenylimino)ethyl)-6-(1-(2,6-dimethylphenyl imino)ethyl)pyridylchromium(III) chloride (Cr5)
3.4. X-ray Crystallographic Studies
3.5. Ethylene Polymerization Procedures
3.5.1. Ethylene Polymerization at 1 Atmosphere Ethylene Pressure
3.5.2. Ethylene Polymerization at 5/10 Atmosphere Ethylene Pressure
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hogan, J.P. Ethylene Polymerization Catalysis over Chromium Oxide. J. Polym. Sci. Part A-1 Polym. Chem. Ed. 1970, 8, 2637–2652. [Google Scholar] [CrossRef]
- Hogan, J.P.; Banks, R.L. Polymers and Production Thereof. U.S. Patent 2825721, 4 March 1958. [Google Scholar]
- McDaniel, M.P. Supported Chromium Catalysts for Ethylene Polymerization. Adv. Catal. 1985, 33, 47–98. [Google Scholar]
- Karol, F.J.; Brown, G.L.; Davison, J.M. Chromocene-Based Catalysts for Ethylene Polymerization: Kinetic Parameters. J. Polym. Sci. Polym. Chem. Ed. 1973, 11, 413–424. [Google Scholar] [CrossRef]
- Groppo, E.; Lamberti, C.; Bordiga, S.; Spoto, A.G.; Zecchina, A. The Structure of Active Centers and the Ethylene Polymerization Mechanism on the Cr/SiO2 Catalyst: A Frontier for the Characterization Methods. Chem. Rev. 2005, 105, 115–183. [Google Scholar] [CrossRef] [PubMed]
- Karapinka, G.L. Verfahren zur Polymerisation von Athylen und Katalysator fur Dasselbe. DE1808388A1, 29 January 1970. [Google Scholar]
- Karapinka, G.L. Polymerization of Ethylene Using Supported Bis-(cyclopentadienyl)chromium(II) Catalysts. U.S. Patent 3709853, 9 January 1973. [Google Scholar]
- Karol, F.J.; Karapinka, G.L.; Wu, C.; Dow, A.W.; Johnson, R.N.; Carrick, W.L. Chromocene catalysts for ethylene polymerization: Scope of the polymerization. J. Polym. Sci. Part A-1 Polym. Chem. 1972, 10, 2621–2637. [Google Scholar] [CrossRef]
- Theopold, K.H. Homogeneous Chromium Catalysts for Olefin Polymerization. Eur. J. Inorg. Chem. 1998, 1998, 15–24. [Google Scholar] [CrossRef]
- Theopold, K.H. Understanding chromium-based olefin polymerization catalysis. Chemtech 1997, 27, 26–32. [Google Scholar]
- Gibson, V.C.; Spitzmesser, S.K. Advances in Non-Metallocene Olefin Polymerization Catalysis. Chem. Rev. 2003, 103, 283–315. [Google Scholar] [CrossRef]
- Tsurugi, H.; Yamamoto, K.; Rochat, R.; Mashima, K. Non-bridged half-metallocene complexes of group 4–6 metals with chelating ligands as well-defined catalysts for α-olefin polymerization. Polym. J. 2015, 47, 2–17. [Google Scholar] [CrossRef]
- MacAdams, L.A.; Kim, W.-K.; Liable-Sands, L.M.; Guzei, I.A.; Rheingold, A.L.; Theopold, K.H. The (Ph)2nacnac Ligand in Organochromium Chemistry. Organometallics 2002, 21, 952–960. [Google Scholar] [CrossRef]
- Dixon, J.T.; Green, M.J.; Hess, F.M.; Morgan, D.H. Advances in selective ethylene trimerization—A critical overview. J. Organomet. Chem. 2004, 689, 3641–3668. [Google Scholar] [CrossRef]
- Gibson, V.C.; Redshaw, C.; Solan, G.A. Bis(imino)pyridines: Surprisingly Reactive Ligands and a Gateway to New Families of Catalysts. Chem. Rev. 2007, 107, 1745–1776. [Google Scholar] [CrossRef] [PubMed]
- Wass, D.F. Chromium-catalysed ethene trimerisation and tetramerisation—Breaking the rules in olefin oligomerisation. Dalton Trans. 2007, 8, 816–819. [Google Scholar] [CrossRef] [PubMed]
- McGuinness, D.S. Olefin Oligomerization via Metallacycles: Dimerization, Trimerization, Tetramerization, and Beyond. Chem. Rev. 2011, 111, 2321–2341. [Google Scholar] [CrossRef]
- Agapie, T. Selective ethylene oligomerization: Recent advances in chromium catalysis and mechanistic investigations. Coord. Chem. Rev. 2011, 255, 861–880. [Google Scholar] [CrossRef]
- Van Leeuwen, P.W.N.M.; Clément, N.D.; Tschan, M.J.-L. New processes for the selective production of 1-octene. Coord. Chem. Rev. 2011, 255, 1499–1517. [Google Scholar] [CrossRef]
- Bryliakov, K.P.; Talsi, E.P. Frontiers of mechanistic studies of coordination polymerization and oligomerization of α-olefins. Coord. Chem. Rev. 2012, 256, 2994–3007. [Google Scholar] [CrossRef]
- Otero, A.; Fernández-Baeza, J.; Lara-Sánchez, A.; Sánchez-Barba, L.F. Metal complexes with heteroscorpionate ligands based on the bis(pyrazol-1-yl)methane moiety: Catalytic chemistry. Coord. Chem. Rev. 2013, 257, 1806–1868. [Google Scholar] [CrossRef]
- Katla, V.; Du, S.; Redshaw, C.; Sun, W.-H. Chromium Complex Precatalysts in Ethylene Oligomerization/Polymerization. Rev. Catal. 2014, 1, 1–14. [Google Scholar]
- Fliedel, C.; Ghisolfi, A.; Braunstein, P. ChemInform Abstract: Functional Short-Bite Ligands: Synthesis, Coordination Chemistry, and Applications of N-Functionalized Bis(diaryl/dialkylphosphino)amine-type Ligands. Chem. Rev. 2016, 116, 9237–9304. [Google Scholar] [CrossRef]
- Alferov, K.A.; Belov, G.P.; Meng, Y. Chromium catalysts for selective ethylene oligomerization to 1-hexene and 1-octene: Recent results. Appl. Catal. A 2017, 542, 71–124. [Google Scholar] [CrossRef]
- Bariashir, C.; Huang, C.; Solan, G.A.; Sun, W.-H. Recent advances in homogeneous chromium catalyst design for ethylene tri-, tetra-, oligo- and polymerization. Coord. Chem. Rev. 2019, 385, 208–229. [Google Scholar] [CrossRef] [Green Version]
- Wass, D.F. Olefin Trimerization Using a Catalyst Comprising a Source of Chromium, Molybdenum or Tungsten and a Ligand Containing at Least One Phosphorous, Arsenic or Antimony Atom Bound to at Least One (Hetero) Hydrocarbyl Group. U.S. Patent US7141633B2, 17 January 2002. [Google Scholar]
- Carter, A.; Cohen, S.A.; Cooley, N.A.; Murphy, A.; Scutt, J.; Wass, D.F. High activity ethylene trimerisation catalysts based on diphosphine ligands. Chem. Commun. 2002, 8, 858–859. [Google Scholar] [CrossRef] [PubMed]
- Emrich, R.; Heinemann, O.; Jolly, P.W.; Krüger, C.; Verhovnik, G.P.J. The Role of Metallacycles in the Chromium-Catalyzed Trimerization of Ethylene. Organometallics 1997, 16, 1511–1513. [Google Scholar] [CrossRef]
- McGuinness, D.S.; Wasserscheid, P.; Keim, W.; Morgan, D.; Dixon, J.T.; Bollmann, A.; Maumela, H.; Hess, F.; Englert, U. First Cr(III)–SNS Complexes and Their Use as Highly Efficient Catalysts for the Trimerization of Ethylene to 1-Hexene. J. Am. Chem. Soc. 2003, 125, 5272–5273. [Google Scholar] [CrossRef]
- McGuinness, D.S.; Wasserscheid, P.; Keim, W.; Hu, C.; Englert, U.; Dixon, J.T.; Grove, C. Novel Cr-PNP complexes as catalysts for the trimerisation of ethylene. Chem. Commun. 2003, 3, 334–335. [Google Scholar] [CrossRef]
- Bollmann, A.; Blann, K.; Dixon, J.T.; Hess, F.M.; Killian, E.; Maumela, H.; McGuinness, D.S.; Morgan, D.H.; Neveling, A.; Otto, S.; et al. Ethylene Tetramerization: A New Route to Produce 1-Octene in Exceptionally High Selectivities. J. Am. Chem. Soc. 2004, 126, 14712–14713. [Google Scholar] [CrossRef]
- Bochmann, M. Cationic Group 4 metallocene complexes and their role in polymerisation catalysis: The chemistry of well defined Ziegler catalysts. J. Chem. Soc. Dalton Trans. 1996, 3, 255–270. [Google Scholar] [CrossRef]
- Kaminsky, W. Highly active metallocene catalysts for olefin polymerization. J. Chem. Soc. Dalton Trans. 1998, 9, 1413–1418. [Google Scholar] [CrossRef]
- McKnight, A.L.; Waymouth, R.M. Group 4 ansa-Cyclopentadienyl-Amido Catalysts for Olefin Polymerization. Chem. Rev. 1998, 98, 2587–2598. [Google Scholar] [CrossRef]
- Resconi, L.; Cavallo, L.; Fait, A.; Piemontesi, F. Selectivity in Propene Polymerization with Metallocene Catalysts. Chem. Rev. 2000, 100, 1253–1346. [Google Scholar] [CrossRef] [PubMed]
- Coates, G.W. Precise Control of Polyolefin Stereochemistry Using Single-Site Metal Catalysts. Chem. Rev. 2000, 100, 1223–1252. [Google Scholar] [CrossRef] [PubMed]
- Alt, H.G.; Köppl, A. Effect of the Nature of Metallocene Complexes of Group IV Metals on Their Performance in Catalytic Ethylene and Propylene Polymerization. Chem. Rev. 2000, 100, 1205–1222. [Google Scholar] [CrossRef] [PubMed]
- McDaniel, M.P. A Review of the Phillips Supported Chromium Catalyst and Its Commercial Use for Ethylene Polymerization. Adv. Catal. 2010, 42, 123–606. [Google Scholar]
- Esteruelas, M.A.; López, A.M.; Méndez, L.; Oliván, M.; Oñate, E. Preparation, Structure, and Ethylene Polymerization Behavior of Bis(imino)pyridyl Chromium(III) Complexes. Organometallics 2003, 22, 395–406. [Google Scholar] [CrossRef]
- Small, B.L.; Carney, M.J.; Holman, D.M.; O’Rourke, C.E.; Halfen, J.A. New Chromium Complexes for Ethylene Oligomerization: Extended Use of Tridentate Ligands in Metal-Catalyzed Olefin Polymerization. Macromolecules 2004, 37, 4375–4386. [Google Scholar] [CrossRef]
- Semikolenova, N.; Zakharov, V.; Echevskaja, L.; Matsko, M.; Bryliakov, K.; Talsi, E. Homogeneous catalysts for ethylene polymerization based on bis(imino)pyridine complexes of iron, cobalt, vanadium and chromium. Catal. Today 2009, 144, 334–340. [Google Scholar] [CrossRef]
- Amolegbe, S.A.; Asma, M.; Zhang, M.; Li, G.; Sun, W.-H. Synthesis, Characterization, and Ethylene Oligomerization and Polymerization by 2-Quinoxalinyl-6-iminopyridine Chromium Chlorides. Aust. J. Chem. 2008, 61, 397–403. [Google Scholar] [CrossRef]
- Xiao, L.; Zhang, M.; Sun, W.-H. Synthesis, characterization and ethylene oligomerization and polymerization of 2-(1H-2-benzimidazolyl)-6-(1-(arylimino)ethyl)pyridylchromium chlorides. Polyhedron 2010, 29, 142–147. [Google Scholar] [CrossRef]
- Chen, Y.; Zuo, W.; Hao, P.; Zhang, S.; Gao, K.; Sun, W.-H. Chromium (III) complexes ligated by 2-(1-isopropyl-2-benzimidazolyl)-6-(1-(arylimino)ethyl)pyridines: Synthesis, characterization and their ethylene oligomerization and polymerization. J. Organomet. Chem. 2008, 693, 750–762. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, C.; Hao, X.; Hu, X.; Sun, W.-H. Accessing highly linear polyethylenes by 2-(1-aryliminoethyl)-7-arylimino-6,6-dimethylcyclopenta[b]pyridylchromium(III) chlorides. RSC Adv. 2016, 6, 91401–91408. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, Y.; Solan, G.A.; Ma, Y.; Hu, X.; Sun, Y.; Sun, W.-H. Vinyl-Polyethylene Waxes with Narrow Dispersity Obtained by Using a Thermally Robust [Bis(imino)trihydroquinolyl]chromium Catalyst. Eur. J. Inorg. Chem. 2017, 36, 4158–4166. [Google Scholar] [CrossRef]
- Huang, C.; Huang, Y.; Ma, Y.; Solan, G.A.; Sun, Y.; Hu, X.; Sun, W.-H. Cycloheptyl-fused N,N,N’-chromium catalysts with selectivity for vinyl-terminated polyethylene waxes: Thermal optimization and polymer functionalization. Dalton Trans. 2018, 47, 13487–13497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.; Du, S.; Solan, G.A.; Sun, Y.; Sun, W.-H. From polyethylene waxes to HDPE using an α,α’-bis(arylimino)-2,3:5,6-bis(pentamethylene)pyridyl-chromium(III) chloride pre-catalyst in ethylene polymerisation. Dalton Trans. 2017, 46, 6948–6957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomov, A.; Chirinos, J.J.; Jones, D.J.; Long, R.J.; Gibson, V.C. Experimental Evidence for Large Ring Metallacycle Intermediates in Polyethylene Chain Growth Using Homogeneous Chromium Catalysts. J. Am. Chem. Soc. 2005, 127, 10166–10167. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Sun, W.-H.; Zhang, S.; Hou, J.; Wedeking, K.; Schultz, S.; Frölich, A.R.; Song, H. Synthesis, Characterization, and Ethylene Oligomerization and Polymerization of [2,6-Bis(2-benzimidazolyl)pyridyl]chromium Chlorides. Organometallics 2006, 25, 1961–1969. [Google Scholar] [CrossRef]
- Zhang, S.; Jie, S.; Shi, Q.; Sun, W.-H. Chromium(III) complexes bearing 2-imino-1,10-phenanthrolines: Synthesis, molecular structures and ethylene oligomerization and polymerization. J. Mol. Catal. A Chem. 2007, 276, 174–183. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, K.; Sun, W.-H. Chromium(III) complexes bearing 2-benzazole-1,10-phenanthrolines: Synthesis, molecular structures and ethylene oligomerization and polymerization. Dalton Trans. 2009, 32, 6354–6363. [Google Scholar] [CrossRef]
- Gao, R.; Liang, T.; Wang, F.; Sun, W.-H. Chromium(III) complexes bearing 2-benzoxazolyl-6-arylimino- pyridines: Synthesis and their ethylene reactivity. J. Organomet. Chem. 2009, 694, 3701–3707. [Google Scholar] [CrossRef]
- Yu, J.; Liu, H.; Zhang, W.; Hao, X.; Sun, W.-H. Access to highly active and thermally stable iron procatalysts using bulky 2-[1-(2,6-dibenzhydryl-4-methylphenylimino)ethyl]-6-[1-(arylimino)ethyl]pyridine Ligands. Chem. Commun. 2011, 47, 3257–3259. [Google Scholar] [CrossRef]
- Cao, X.; He, F.; Zhao, W.; Cai, Z.; Hao, X.; Shiono, T.; Redshaw, C.; Sun, W.-H. 2-[1-(2,6-Dibenzhydryl-4- chlorophenylimino)ethyl]-6-[1-(arylimino)ethyl]pyridyliron(II) dichlorides: Synthesis, characterization and ethylene polymerization behavior. Polymer 2012, 53, 1870–1880. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, Y.; Suo, H.; Solan, G.A.; Liang, T.; Sun, W.-H. Co-catalyst effects on the thermal stability/activity of N,N,N-Co ethylene polymerization Catalysts Bearing Fluoro-Substituted N-2,6-dibenzhydrylphenyl groups. Appl. Organomet. Chem. 2019, 33, 5134. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, R.; Han, M.; Yang, W.; Liang, T.; Sun, W.-H.; Tongling, L. 4,4’-Difluorobenzhydryl- modified bis(imino)-pyridyliron (II) chlorides as thermally stable precatalysts for strictly linear polyethylenes with narrow dispersities. Dalton Trans. 2020, 49, 7384–7396. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, B.; Liang, T.; Redshaw, C.; Li, Y.; Sun, W.-H. Synthesis, characterization and catalytic behavior toward ethylene of 2-[1-(4,6-dimethyl-2-benzhydryl-phenylimino)ethyl]-6-[1-(arylimino)ethyl]pyridyl metal (iron or cobalt) chlorides. Dalton Trans. 2013, 42, 9188–9197. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, S.; Du, S.; Guo, C.-Y.; Hao, X.; Sun, W.-H. 2-(1-(2,4-Bis((di(4-fluorophenyl)methyl)-6- methylphenylimino)ethyl)-6-(1-(arylimino)ethyl)pyridylmetal (iron or cobalt) Complexes: Synthesis, Characterization, and Ethylene Polymerization Behavior. Macromol. Chem. Phys. 2014, 215, 1797–1809. [Google Scholar] [CrossRef]
- Mahmood, Q.; Guo, J.; Zhang, W.; Ma, Y.; Liang, T.; Sun, W.-H. Concurrently Improving the Thermal Stability and Activity of Ferrous Precatalysts for the Production of Saturated/Unsaturated Polyethylene. Organometallics 2018, 37, 957–970. [Google Scholar] [CrossRef]
- Mahmood, Q.; Yue, E.; Guo, J.; Zhang, W.; Ma, Y.; Hao, X.; Sun, W.-H. Nitro-functionalized bis(imino)pyridylferrous chlorides as thermo-stable precatalysts for linear polyethylenes with high molecular weights. Polymer 2018, 159, 124–137. [Google Scholar] [CrossRef]
- Huang, C.; Vignesh, A.; Bariashir, C.; Mahmood, Q.; Ma, Y.; Sun, Y.; Sun, W.-H. Producing Highly Linear Polyethylenes by Using t-butyl-Functionalized 2,6-bis(imino)pyridylchromium (III) Chlorides. J. Polym. Sci. Part. A Polym. Chem. 2019, 57, 1049–1058. [Google Scholar] [CrossRef]
- Huang, C.; Vignesh, A.; Bariashir, C.; Ma, Y.; Sun, Y.; Sun, W.-H. Achievement of strictly linear ultra-high molecular weight polyethylene with narrow dispersity by dint of nitro-enhanced 2,6-bis(imino)- pyridylchromium chloride complexes. New J. Chem. 2019, 43, 11307–11315. [Google Scholar] [CrossRef]
- Gansukh, B.; Zhang, Q.; Flisak, Z.; Liang, T.; Ma, Y.; Sun, W.-H. The chloro-substituent enhances performance of 2,4-bis(imino)pyridylchromium catalysts yielding highly linear polyethylene. Appl. Organomet. Chem. 2020, 34, 5471. [Google Scholar] [CrossRef]
- Britovsek, G.J.P.; Gibson, V.C.; Hoarau, O.D.; Spitzmesser, S.K.; White, A.J.P.; Williams, D.J. Iron and Cobalt Ethylene Polymerization Catalysts: Variations on the Central Donor. Inorg. Chem. 2003, 42, 3454–3465. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.-H.; Tang, X.; Gao, T.; Wu, B.; Zhang, W.; Ma, H. Synthesis, Characterization, and Ethylene Oligomerization and Polymerization of Ferrous and Cobaltous 2-(Ethylcarboxylato)-6-iminopyridyl Complexes. Organometallics 2004, 23, 5037–5047. [Google Scholar] [CrossRef]
- Gibson, V.C.; Redshaw, C.; Solan, G.A.; White, A.J.P.; Williams, D.J. Aluminum Alkyl-Mediated Route to Novel N,N,O-Chelates for Five-Coordinate Iron(II) Chloride Complexes: Synthesis, Structures, and Ethylene Polymerization Studies. Organometallics 2007, 26, 5119–5123. [Google Scholar] [CrossRef]
- Smit, T.M.; Tomov, A.K.; Britovsek, G.J.P.; Gibson, V.C.; White, A.J.P.; Williams, D.J. The effect of imine-carbon substituents in bis(imino)pyridine-based ethylene polymerisation catalysts across the transition series. Catal. Sci. Technol. 2012, 2, 643–655. [Google Scholar] [CrossRef]
- Semikolenova, N.V.; Sun, W.-H.; Soshnikov, I.E.; Matsko, M.A.; Kolesova, O.V.; Zakharov, V.A.; Bryliakov, K.P. Origin of “Multisite-like” Ethylene Polymerization Behavior of the Single-Site Nonsymmetrical Bis(imino)pyridine Iron(II) Complex in the Presence of Modified Methylaluminoxane. ACS Catal. 2017, 7, 2868–2877. [Google Scholar] [CrossRef]
- Gates, D.P.; Svejda, S.K.; Onate, E.; Killian, C.M.; Johnson, L.K.; White, P.S.; Brookhart, M. Synthesis of Branched Polyethylene Using (α-Diimine)nickel (II) Catalysts: Influence of Temperature, Ethylene Pressure, and Ligand Structure on Polymer Properties. Macromolecules 2000, 33, 2320–2334. [Google Scholar] [CrossRef]
- Lee, L.-S.; Ou, H.-J.; Hsu, H.-L. The experiments and correlations of the solubility of ethylene in toluene solvent. Fluid Phase Equilib. 2005, 231, 221–230. [Google Scholar] [CrossRef]
- Popeney, C.S.; Rheingold, A.L.; Guan, Z. Nickel(II) and Palladium(II) Polymerization Catalysts Bearing a Fluorinated Cyclophane Ligand: Stabilization of the Reactive Intermediate(1). Organometallics 2009, 28, 4452–4463. [Google Scholar] [CrossRef]
- Britovsek, G.J.P.; Bruce, M.; Gibson, V.C.; Kimberley, B.S.; Maddox, P.J.; Mastroianni, S.; McTavish, S.J.; Redshaw, C.; Solan, G.A.; Strömberg, S.; et al. Iron and Cobalt Ethylene Polymerization Catalysts Bearing 2,6-Bis(Imino)Pyridyl Ligands: Synthesis, Structures, and Polymerization Studies. J. Am. Chem. Soc. 1999, 121, 8728–8740. [Google Scholar] [CrossRef]
- Bariashir, C.; Wang, Z.; Ma, Y.; Vignesh, A.; Hao, X.; Sun, W.-H. Finely Tuned α,α’-Bis(arylimino)-2,3:5,6- bis(pentamethylene)pyridine-Based Practical Iron Precatalysts for Targeting Highly Linear and Narrow Dispersive Polyethylene Waxes with Vinyl Ends. Organometallics 2019, 38, 4455–4470. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, N.; Xiang, J.; Solan, G.A.; Suo, H.; Ma, Y.; Liang, T.; Sun, W.-H. Bis-cycloheptyl-fused bis(imino)pyridine-cobalt catalysts for PE wax formation: Positive effects of fluoride substitution on catalytic performance and thermal stability. Dalton Trans. 2020, 49, 9425–9437. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G. SHELXT- integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, C71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Spek, A.L. Structure validation in chemical crystallography. Acta Crystallogr. Sect. D Biol. Crystallogr. 2009, D65, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Van Der Sluis, P.; Spek, A.L. BYPASS: An effective method for the refinement of crystal structures containing disordered solvent regions. Acta Crystallogr. Sect. A Found. Adv. 1990, A46, 194–201. [Google Scholar] [CrossRef]










| Cr2 | Cr4 | Cr2 | Cr4 | ||
|---|---|---|---|---|---|
| bond lengths (Å) | bond angles (°) | ||||
| Cr1–Cl1 | 2.2900 (18) | 2.2757 (12) | Cl1–Cr1–N1 | 174.81 (17) | 176.21 (10) |
| Cr1–Cl2 | 2.2788 (19) | 2.3295 (12) | Cl1–Cr1–N2 | 107.04 (15) | 105.71 (9) |
| Cr1–Cl3 | 2.3217 (19) | 2.3278 (12) | Cl1–Cr1–N3 | 99.53 (14) | 99.22 (11) |
| Cr1–N1 | 2.003 (5) | 1.985 (3) | Cl2–Cr1–N1 | 91.48 (15) | 82.50 (10) |
| Cr1–N2 | 2.157 (5) | 2.132 (3) | Cl2–Cr1–N2 | 89.18 (15) | 88.62 (9) |
| Cr1–N3 | 2.133 (5) | 2.136 (3) | Cl2–Cr1–N3 | 86.33 (14) | 89.58 (10) |
| C2–N2 | 1.290 (8) | 1.297 (5) | Cl3–Cr1–N1 | 85.08 (15) | 87.23 (10) |
| C8–N3 | 1.299 (7) | 1.281 (6) | Cl3–Cr1–N2 | 91.29 (15) | 88.38 (9) |
| bond angles (°) | Cl3–Cr1–N3 | 91.61 (14) | 88.98 (10) | ||
| Cl1–Cr1–Cl2 | 92.31 (8) | 96.57 (5) | N1–Cr1–N2 | 76.5 (2) | 77.97 (13) |
| Cl1–Cr1–Cl3 | 91.04 (8) | 93.74 (4) | N1–Cr1–N3 | 77.2 (2) | 77.12 (14) |
| Cl2–Cr1–Cl3 | 176.31 (8) | 169.69 (5) | N2–Cr1–N3 | 153.20 (19) | 155.05 (14) |
| Entry | Cat. | Al:Cr | T, °C | t, min | PE, g | Activity b | Mwc | Mw/Mnc | Tmd, °C |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Cr4 | 2000 | 30 | 30 | 1.08 | 1.08 | 1.05 | 1.68 | 120.6 |
| 2 | Cr4 | 2500 | 30 | 30 | 1.97 | 1.97 | 1.11 | 1.71 | 121.1 |
| 3 | Cr4 | 3000 | 30 | 30 | 3.27 | 3.27 | 1.15 | 1.46 | 122.0 |
| 4 | Cr4 | 3250 | 30 | 30 | 5.14 | 5.14 | 1.17 | 1.79 | 122.8 |
| 5 | Cr4 | 3500 | 30 | 30 | 5.46 | 5.46 | 1.22 | 1.89 | 122.9 |
| 6 | Cr4 | 3750 | 30 | 30 | 4.78 | 4.78 | 1.09 | 1.69 | 122.7 |
| 7 | Cr4 | 4000 | 30 | 30 | 2.88 | 2.88 | 1.04 | 1.52 | 122.5 |
| 8 | Cr4 | 3500 | 40 | 30 | 5.64 | 5.64 | 1.48 | 1.94 | 122.0 |
| 9 | Cr4 | 3500 | 50 | 30 | 7.52 | 7.52 | 1.51 | 2.09 | 122.5 |
| 10 | Cr4 | 3500 | 60 | 30 | 10.03 | 10.03 | 1.61 | 2.25 | 123.5 |
| 11 | Cr4 | 3500 | 70 | 30 | 6.24 | 6.24 | 1.44 | 2.01 | 121.6 |
| 12 | Cr4 | 3500 | 60 | 05 | 1.66 | 9.96 | 1.02 | 1.67 | 120.8 |
| 13 | Cr4 | 3500 | 60 | 15 | 4.30 | 8.60 | 1.18 | 1.72 | 121.2 |
| 14 | Cr4 | 3500 | 60 | 45 | 10.24 | 6.83 | 1.61 | 2.16 | 121.1 |
| 15 | Cr4 | 3500 | 60 | 60 | 10.54 | 5.27 | 1.67 | 2.18 | 120.7 |
| 16 e | Cr4 | 3500 | 60 | 30 | 4.88 | 4.88 | 0.98 | 1.86 | 121.3 |
| 17 f | Cr4 | 3500 | 60 | 30 | Trace | - | - | - | - |
| Entry | Precatalyst | PE, g | Activity b | Mwc | Mw/Mnc | Tmd, °C |
|---|---|---|---|---|---|---|
| 1 | Cr1 | 8.28 | 8.28 | 1.01 | 1.62 | 121.8 |
| 2 | Cr2 | 6.86 | 6.86 | 1.74 | 1.99 | 122.3 |
| 3 | Cr3 | 6.16 | 6.16 | 1.78 | 2.03 | 122.9 |
| 4 | Cr4 | 10.03 | 10.03 | 1.61 | 2.25 | 123.5 |
| 5 | Cr5 | 7.27 | 7.27 | 2.23 | 2.22 | 125.2 |
| Entry | Cat. | Al:Cr | T, °C | t, min | PE, g | Activity b | Mwc | Mw/Mnc | Tmd, °C |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Cr4 | 2000 | 30 | 30 | 5.18 | 5.18 | 0.77 | 1.71 | 119.9 |
| 2 | Cr4 | 2500 | 30 | 30 | 7.44 | 7.44 | 1.09 | 2.01 | 120.0 |
| 3 | Cr4 | 3000 | 30 | 30 | 12.14 | 12.14 | 1.27 | 2.14 | 120.9 |
| 4 | Cr4 | 3500 | 30 | 30 | 15.53 | 15.53 | 1.36 | 1.91 | 121.0 |
| 5 | Cr4 | 3750 | 30 | 30 | 17.51 | 17.51 | 1.38 | 1.95 | 121.7 |
| 6 | Cr4 | 4000 | 30 | 30 | 20.14 | 20.14 | 1.43 | 1.97 | 122.4 |
| 7 | Cr4 | 4250 | 30 | 30 | 13.27 | 13.27 | 1.05 | 1.89 | 120.8 |
| 8 | Cr4 | 4500 | 30 | 30 | 11.14 | 11.14 | 0.78 | 1.62 | 120.5 |
| 9 | Cr4 | 4000 | 20 | 30 | 13.28 | 13.28 | 0.89 | 1.76 | 120.3 |
| 10 | Cr4 | 4000 | 40 | 30 | 17.84 | 17.84 | 1.20 | 2.07 | 122.0 |
| 11 | Cr4 | 4000 | 50 | 30 | 13.63 | 13.63 | 1.07 | 1.80 | 121.5 |
| 12 | Cr4 | 4000 | 60 | 30 | 11.76 | 11.76 | 1.06 | 1.73 | 120.4 |
| 13 | Cr4 | 4000 | 30 | 05 | 3.03 | 18.18 | 0.67 | 1.39 | 119.1 |
| 14 | Cr4 | 4000 | 30 | 15 | 9.52 | 19.04 | 0.73 | 1.52 | 120.3 |
| 15 | Cr4 | 4000 | 30 | 45 | 22.31 | 14.87 | 1.48 | 1.88 | 120.4 |
| 16 | Cr4 | 4000 | 30 | 60 | 22.79 | 11.39 | 1.55 | 2.42 | 120.7 |
| 17 e | Cr4 | 4000 | 30 | 30 | 8.56 | 8.56 | 0.76 | 1.71 | 119.4 |
| 18 f | Cr4 | 4000 | 30 | 30 | Trace | - | - | - | - |
| Entry | Precatalyst | PE, g | Activity b | Mwc | Mw/Mnc | Tmd, °C |
|---|---|---|---|---|---|---|
| 1 | Cr1 | 8.93 | 8.93 | 1.01 | 1.92 | 120.4 |
| 2 | Cr2 | 10.78 | 10.78 | 1.59 | 2.12 | 121.3 |
| 3 | Cr3 | 7.59 | 7.59 | 1.80 | 2.01 | 123.5 |
| 4 | Cr4 | 20.14 | 20.14 | 1.43 | 1.97 | 122.4 |
| 5 | Cr5 | 12.67 | 12.67 | 2.49 | 2.27 | 125.0 |
| Complex | Cr2 | Cr4 |
|---|---|---|
| CCDC No. | 2,002,415 | 2,002,416 |
| Empirical formula | C51H46Cl3CrFN3 | C50H44Cl3CrFN3 |
| Formula weight | 878.25 | 864.23 |
| Temperature (K) | 169.99 (10) | 170.00 (15) |
| Wavelength (Å) | 0.71073 | 0.71073 |
| Crystal system | Monoclinic | Monoclinic |
| Space group | P21/c | P21/c |
| a (Å) | 15.9706 (6) | 27.1328 (5) |
| b (Å) | 39.1059 (18) | 27.2064 (4) |
| c (Å) | 15.2664 (6) | 17.0515 (3) |
| α (°) | 90 | 90 |
| β (°) | 91.412 (4) | 104.612 (2) |
| γ (°) | 90 | 90 |
| Volume (Å3) | 9531.7 (7) | 12180.1 (4) |
| Z | 4 | 4 |
| Dcalcd (g cm−3) | 1.224 | 0.943 |
| µ (mm−1) | 3.832 | 2.922 |
| F (000) | 3656.0 | 3592.0 |
| Crystal size (mm) | 0.53 × 0.29 × 0.18 | 0.20 × 0.15 × 0.09 |
| 2θ Range (°) | 4.52 to 151.222 | 4.678 to 151.134 |
| Limiting indices | −19 ≤ h ≤ 19, −48 ≤ k ≤ 47, −19 ≤ l ≤ 18 | −33 ≤ h ≤ 25, −32 ≤ k ≤ 34, −21 ≤ l ≤ 21 |
| No. of rflns collected | 78028 | 103413 |
| No. unique rflns [R(int)] | 18,707 [Rint = 0.0992, Rsigma = 0.0691] | 24,269 [Rint = 0.0847, Rsigma = 0.0633] |
| Completeness to θ (%) | 100% | 100% |
| Data/restraints/parameters | 18,707/81/1082 | 24,269/72/1055 |
| The goodness of fit on F2 | 1.349 | 1.044 |
| Final R indices [I > 2σ(I)] | R1 = 0.1094, wR2 = 0.3181 | R1 = 0.0776, wR2 = 0.2220 |
| R Indices (all data) | R1 = 0.1436, wR2 = 0.3634 | R1 = 0.1155, wR2 = 0.2581 |
Sample Availability: Samples of the compounds are available from the authors if readers make requirements. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gansukh, B.; Zhang, Q.; Bariashir, C.; Vignesh, A.; Ma, Y.; Liang, T.; Sun, W.-H. Unifying Molecular Weights of Highly Linear Polyethylene Waxes through Unsymmetrical 2,4-Bis(imino)pyridylchromium Chlorides. Molecules 2020, 25, 5584. https://doi.org/10.3390/molecules25235584
Gansukh B, Zhang Q, Bariashir C, Vignesh A, Ma Y, Liang T, Sun W-H. Unifying Molecular Weights of Highly Linear Polyethylene Waxes through Unsymmetrical 2,4-Bis(imino)pyridylchromium Chlorides. Molecules. 2020; 25(23):5584. https://doi.org/10.3390/molecules25235584
Chicago/Turabian StyleGansukh, Badral, Qiuyue Zhang, Chantsalnyam Bariashir, Arumugam Vignesh, Yanping Ma, Tongling Liang, and Wen-Hua Sun. 2020. "Unifying Molecular Weights of Highly Linear Polyethylene Waxes through Unsymmetrical 2,4-Bis(imino)pyridylchromium Chlorides" Molecules 25, no. 23: 5584. https://doi.org/10.3390/molecules25235584
APA StyleGansukh, B., Zhang, Q., Bariashir, C., Vignesh, A., Ma, Y., Liang, T., & Sun, W. -H. (2020). Unifying Molecular Weights of Highly Linear Polyethylene Waxes through Unsymmetrical 2,4-Bis(imino)pyridylchromium Chlorides. Molecules, 25(23), 5584. https://doi.org/10.3390/molecules25235584