Newly Generated Atractylon Derivatives in Processed Rhizomes of Atractylodes macrocephala Koidz
Abstract
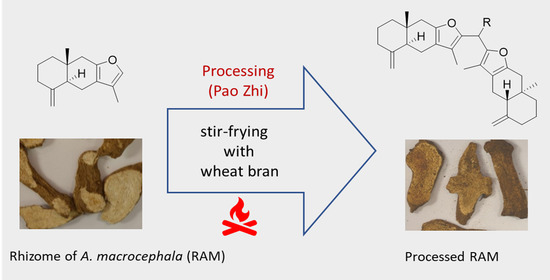
:1. Introduction
2. Results and Discussion
2.1. Structure Elucidation
2.2. Proposed Mechanism for the Formation of Compounds 1–5
2.3. Biological Activities of Compounds 1–5
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material and Preparation
3.3. Extraction and Isolation
3.4. Characterization of Compounds 1–5
3.5. Biological Activity Assays
3.5.1. Cytotoxicity Assay
3.5.2. Antimicrobial Assays
3.5.3. Antileishmanial Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhu, B.; Zhang, Q.-L.; Hua, J.-W.; Cheng, W.-L.; Qin, L.-P. The traditional uses, phytochemistry, and pharmacology of Atractylodes macrocephala Koidz.: A review. J. Ethnopharmacol. 2018, 226, 143–167. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Li, Y.; Xu, Y.; Zhu, Y. Anti-aging traditional Chinese medicine: Potential mechanisms involving AMPK pathway and calorie restriction based on “medicine-food homology” theory. China J. Chin. Mater. Med. 2016, 41, 1144–1151. [Google Scholar] [CrossRef]
- Li, Y.; Li, W.; Wang, L.; Shao, C.; Zhao, J.; Liu, J. Summary of research on bran fried atractylodes. Shandong Zhong Yi Yao Da Xue 2018, 42, 182–185. [Google Scholar]
- Wang, C.S. The processing of Atractylodes macrocephala by stir-frying with wheat bran. Zhong Yao Tong Bao (Beijing, China: 1981) 1983, 8, 18–19. [Google Scholar]
- Zhao, J.; Wang, M.; Avula, B.; Zhong, L.; Song, Z.; Xu, Q.; Li, S.; Ibrahim, M.A. Effect of Processing on the Traditional Chinese Herbal Medicine Flos Lonicerae: An NMR-based Chemometric Approach. Planta Med. 2015, 81, 754–764. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.; Yuan, S. Dictionary of Chinese Herbal Processing Science; Shanghai Science and Technology Press: Shanghai, China, 2005; p. 102. [Google Scholar]
- State Pharmacopoeia Commission of the PRC. Pharmacopoeia of the People’s Republic of China. 1 (2015); China Medical Science Press: Beijing, China, 2015. [Google Scholar]
- Hoang, L.S.; Tran, M.H.; Lee, J.-S.; Ngo, Q.M.T.; Woo, M.H.; Min, B.S. Inflammatory Inhibitory Activity of Sesquiterpenoids from Atractylodes macrocephala Rhizomes. Chem. Pharm. Bull. 2016, 64, 507–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Zhang, Y.; Wang, Z.; Zhu, J.; Tian, Y.; Chen, B. Quantitative analysis of atractylenolide I in rat plasma by LC–MS/MS method and its application to pharmacokinetic study. J. Pharm. Biomed. Anal. 2012, 58, 172–176. [Google Scholar] [CrossRef]
- Cai, B.; Cai, H.; Xu, Z.; Luo, S.; Zhang, W.; Cao, G.; Liu, X.; Lou, Y.; Ma, X.; Qin, K. Study on chemical fingerprinting of crude and processed Atractylodes macrocephala from different locations in Zhejiang province by reversed-phase high-performance liquid chromatography coupled with hierarchical cluster analysis. Pharmacogn. Mag. 2012, 8, 300–307. [Google Scholar] [CrossRef] [Green Version]
- Cao, G.; Xu, Z.; Wu, X.; Li, Q.; Chen, X. Capture and identification of the volatile components in crude and processed herbal medicines through on-line purge and trap technique coupled with GC × GC-TOF MS. Nat. Prod. Res. 2014, 28, 1607–1612. [Google Scholar] [CrossRef]
- Gu, S.; Li, L.; Huang, H.; Wang, B.; Zhang, T. Antitumor, Antiviral, and Anti-Inflammatory Efficacy of Essential Oils from Atractylodes macrocephala Koidz. Produced with Different Processing Methods. Molecules 2019, 24, 2956. [Google Scholar] [CrossRef] [Green Version]
- Shan, G.-S.; Zhang, L.; Zhao, Q.-M.; Xiao, H.-B.; Zhuo, R.-J.; Xu, G.; Jiang, H.; You, X.-M.; Jia, T.-Z. Metabolomic study of raw and processed Atractylodes macrocephala Koidz by LC–MS. J. Pharm. Biomed. Anal. 2014, 98, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Shan, C.; Wen, H.; Li, W.; Wu, H. UFLC/Q-TOF-MS based analysis on material base of Atractylodes macrocephalae rhizoma stir-fried with wheat bran. Zhongguo Zhongyao Za Zhi 2013, 38, 1929–1933. [Google Scholar]
- Zhao, C.-X.; He, C. Preparative isolation and purification of atractylon and atractylenolide III from the Chinese medicinal plant atractylodes macrocephala by high-speed counter-current chromatography. J. Sep. Sci. 2006, 29, 1630–1636. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, L.; Ran, X.; Dou, D.; Li, B.; Yang, B.; Li, W.; Koike, K.; Kuang, H. What caused the changes in the usage of Atractylodis Macrocephalae Rhizoma from ancient to current times? J. Nat. Med. 2015, 70, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Wu, Q.; Gao, C.; Gai, C.; Yuan, D.; Fu, H. Chemical constituents from the fruit calyx of Physalis alkekengi var. francheti. J. Chin. Pharm. Sci. 2015, 24, 600–606. [Google Scholar] [CrossRef]
- Jiang, D.; Peterson, D.G. Identification of bitter compounds in whole wheat bread. Food Chem. 2013, 141, 1345–1353. [Google Scholar] [CrossRef]
- Hasada, K.; Yoshida, T.; Yamazaki, T.; Sugimoto, N.; Nishimura, T.; Nagatsu, A.; Mizukami, H. Quantitative determination of atractylon in Atractylodis Rhizoma and Atractylodis Lanceae Rhizoma by 1H-NMR spectroscopy. J. Nat. Med. 2010, 64, 161–166. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, C.; Sun, T.-M.; Ran, X.-K.; Kang, T.-G.; Dou, D. Two new compounds from Atractylodes macrocephala with neuroprotective activity. J. Asian Nat. Prod. Res. 2016, 19, 35–41. [Google Scholar] [CrossRef]
- Ding, H.-Y.; Liu, M.-Y.; Chang, W.-L.; Lin, H.-C. New sesquiterpenoids from the rhizomes of Atractylodes macrocephala. Chin. Pharm. J. 2005, 57, 37–42. [Google Scholar]
- Gupta, J.; Ali, M.; Pillai, K.K.; Velasco-Negueruela, A.; Pérez-Alonso, M.J.; Contreras, F.Ó. The Occurrence of Ishwarane and Ishwarone in the Roof Oil of Corallocarpus epigaeus Benth. ex Hook. f. J. Essent. Oil Res. 1997, 9, 667–672. [Google Scholar] [CrossRef]
- Bohlmann, F.; Suwita, A. New sesquiterpenes from Peteravenia schultzii. Phytochemistry 1978, 17, 567–568. [Google Scholar] [CrossRef]
- Stevenson, L.; Phillips, F.; O’Sullivan, K.; Walton, J. Wheat bran: Its composition and benefits to health, a European perspective. Int. J. Food Sci. Nutr. 2012, 63, 1001–1013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Lyons-Hart, J.; Banyasz, J.; Shafer, K. Real-time evolved gas analysis by FTIR method: An experimental study of cellulose pyrolysis. Fuel 2001, 80, 1809–1817. [Google Scholar] [CrossRef]
- Wang, S.; Guo, X.; Liang, T.; Zhou, Y.; Luo, Z. Mechanism research on cellulose pyrolysis by Py-GC/MS and subsequent density functional theory studies. Bioresour. Technol. 2012, 104, 722–728. [Google Scholar] [CrossRef]
- Yin, H.; Wang, Z.; Wang, L.; Zhou, A.-Z.; Li, Q.-L.; Cheng, Z. Simultaneous determination of atractylone, atractylenolide Ⅰ, Ⅱ, Ⅲ in Atractylodes macrocephala by HPLC-wavelength switching method. J. Tradit. Chin. Med. 2013, 1, 235–238. [Google Scholar]
- Yuan, H.; Zhao, J.; Wang, M.; Khan, S.I.; Zhai, C.; Xu, Q.; Huang, J.; Peng, C.; Xiong, G.; Wang, W.; et al. Benzophenone glycosides from the flower buds of Aquilaria sinensis. Fitoterapia 2017, 121, 170–174. [Google Scholar] [CrossRef]
- Auker, K.M.; Coleman, C.M.; Wang, M.; Avula, B.; Bonnet, S.L.; Kimble, L.; Mathison, B.D.; Chew, B.P.; Ferreira, D. Structural Characterization of Cranberry Arabinoxyloglucan Oligosaccharides. J. Nat. Prod. 2019, 82, 606–620. [Google Scholar] [CrossRef]
- Jain, S.K.; Sahu, R.; Walker, L.A.; Tekwani, B. A Parasite Rescue and Transformation Assay for Antileishmanial Screening Against Intracellular Leishmania donovani Amastigotes in THP1 Human Acute Monocytic Leukemia Cell Line. J. Vis. Exp. 2012, 70, e4054. [Google Scholar] [CrossRef] [Green Version]
- Sheridan, H.; Kopp, B.; Krenn, L.; Guo, D.; Sendker, J. Traditional Chinese herbal medicine preparation: Invoking the butterfly effect. Science 2015, 350, S64–S66. [Google Scholar]
- Zhao, Z.; Liang, Z.; Chan, K.; Lu, G.; Lee, E.L.M.; Chen, H.; Li, L. A Unique Issue in the Standardization of Chinese Materia Medica: Processing. Planta Med. 2010, 76, 1975–1986. [Google Scholar] [CrossRef]


| No. * | 1 | 2 | 3 | 4 | 5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| δC | δH | δC | δH | δC | δH | δC | δH | δC | δH | |
| 1, 1′, (1′′) | 42.0 (t) | 1.47, 1.68 (m, 4H) | 41.9/42.0 (t) | 1.49, 1.67 (m, 4H) | 42.0 (t) | 1.47, 1.66 (m, 4H) | 41.9 (t) | 1.48, 1.65 (m, 4H) | 42.0 (t) | 1.47, 1.67 (m, 6H) |
| 2, 2′, (2′′) | 23.6 (t) | 1.57, 1.63 (m, 4H) | 23.6 (t) | 1.56, 1.63 (m, 4H) | 23.6 (t) | 1.55, 1.64 (m, 4H) | 23.6 (t) | 1.53, 1.63 (m, 4H) | 23.6 (t) | 1.54, 1.64 (m, 6H) |
| 3, 3′, (3′′) | 37.3 (t) | 2.02, 2.37 (m, 4H) | 37.4 (t) | 2.02, 2.37 (m, 4H) | 37.3 (t) | 2.02, 2.38 (m, 4H) | 37.3 (t) | 2.02, 2.38 (m, 4H) | 37.3/36.7 (t) | 2.02, 2.37 (m, 6H) |
| 4, 4′, (4′′) | 150.0 (s) | 150.0 (s) | 149.9 (s) | 149.9 (s) | 149.9/150.0 (s) | |||||
| 5, 5′, (5′′) | 45.7 (d) | 2.10 (m, 2H) | 45.7/45.8 (d) | 2.10 (m, 2H) | 45.7 (d) | 2.10 (m, 2H) | 45.6/45.7 (d) | 2.09 (m, 2H) | 45.6/45.7 (d) | 2.10 (m, 3H) |
| 6, 6′, (6′′) | 21.2 (t) | 2.25, 2.32 (m, 4H) | 21.1 (t) | 2.25, 2.32 (m, 4H) | 21.1 (t) | 2.27, 2.34 (m, 4H) | 21.1 (t) | 2.25, 2.32 (m, 4H) | 21.1 (t) | 2.25, 2.32 (m, 6H) |
| 7, 7′, (7′′) | 116.6 (s) | 116.6 (s) | 116.8 (s) | 116.8 (s) | 116.8/116.7 (s) | |||||
| 8, 8′, (8′′) | 146.9 (s) | 146.6 (s) | 147.6 (s) | 147.6 (s) | 147.3/147.0 (s) | |||||
| 9, 9′, (9′′) | 39.2 (t) | 2.41 (m, 4H) | 39.2/39.3 (t) | 2.42 (m, 4H) | 39.2 (t) | 2.42 (m, 4H) | 39.2 (t) | 2.40 (m, 4H) | 39.2/39.1 (t) | 2.40/2.39 (m, 6H) |
| 10, 10′, (10′′) | 36.7 (s) | 36.7 (s) | 36.7 (s) | 36.7 (s) | 36.7/36.4 (s) | |||||
| 11, 11′, (11′′) | 114.3 (s) | 113.0 (s) | 115.2 (s) | 115.2 (s) | 115.1/114.9 (s) | |||||
| 12, 12′, (12′′) | 150.0 (s) | 149.1 (s) | 144.7 (s) | 144.5 (s) | 144.8/144.3 (s) | |||||
| 13, 13′, (13′′) | 8.0 (q) | 1.87 (s, 6H) | 7.8/8.0 (q) | 1.76/1.82 (s, 6H) | 7.8/7.9 (q) | 1.80/1.81 (s, 6H) | 7.8/7.9 (q) | 1.79/1.80 (s, 6H) | 7.8/7.9/8.0 (q) | 1.74/1.76/1.82 (s, 9H) |
| 14, 14′, (14′′) | 17.6 (q) | 0.76 (s, 6H) | 17.6/17.7 (q) | 0.75/0.77 (s, 6H) | 17.6/17.7 (q) | 0.75/0.76 (s, 6H) | 17.6/17.7 (q) | 0.75/0.76 (s, 6H) | 17.6/17.7 (q) | 0.75/0.76 (s, 9H) |
| 15, 15′, (15′′) | 107.1 (t) | 4.68, 4.84 (d, 4H) | 107.1 (t) | 4.68, 4.84 (d, 4H) | 107.1 (t) | 4.68, 4.84 (d, 4H) | 107.2 (t) | 4.67, 4.83 (d, 4H) | 107.1/107.2 (t) | 4.67, 4.83 (d, 6H) |
| 16 | 23.9 (t) | 3.82 (s, 2H) | 30.1 (d) | 4.17 (q, 1H) | 36.1 (d) | 5.47 (s, 1H) | 36.2 (d) | 5.44 (s, 1H) | 36.2 (d) | 5.42 (s, 1H) |
| 17 | 18.1 (q) | 1.57 (d, 3H) | 153.1 (s) | 153.2 (s) | 151.5 (s) | |||||
| 18 | 107.2 (d) | 6.07 (d, 1H) | 108.1 (d) | 6.02 (d, 1H) | 107.9 (d) | 5.93 (d, 1H) | ||||
| 19 | 110.2 (d) | 6.30 (dd, 1H) | 108.7 (d) | 6.20 (d, 1H) | 106.5 (d) | 5.89 (d, 1H) | ||||
| 20 | 141.4 (d) | 7.35 (d, 1H) | 153.0 (s) | 151.4 (s) | ||||||
| 21 | 57.6 (t) | 4.55 (s, 2H) | 25.8 (t) | 3.86 (s, 2H) | ||||||
Sample Availability: Samples of compounds 1–5 are available from the authors. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhai, C.; Zhao, J.; Chittiboyina, A.G.; Meng, Y.; Wang, M.; Khan, I.A. Newly Generated Atractylon Derivatives in Processed Rhizomes of Atractylodes macrocephala Koidz. Molecules 2020, 25, 5904. https://doi.org/10.3390/molecules25245904
Zhai C, Zhao J, Chittiboyina AG, Meng Y, Wang M, Khan IA. Newly Generated Atractylon Derivatives in Processed Rhizomes of Atractylodes macrocephala Koidz. Molecules. 2020; 25(24):5904. https://doi.org/10.3390/molecules25245904
Chicago/Turabian StyleZhai, Chunmei, Jianping Zhao, Amar G. Chittiboyina, Yonghai Meng, Mei Wang, and Ikhlas A. Khan. 2020. "Newly Generated Atractylon Derivatives in Processed Rhizomes of Atractylodes macrocephala Koidz" Molecules 25, no. 24: 5904. https://doi.org/10.3390/molecules25245904
APA StyleZhai, C., Zhao, J., Chittiboyina, A. G., Meng, Y., Wang, M., & Khan, I. A. (2020). Newly Generated Atractylon Derivatives in Processed Rhizomes of Atractylodes macrocephala Koidz. Molecules, 25(24), 5904. https://doi.org/10.3390/molecules25245904