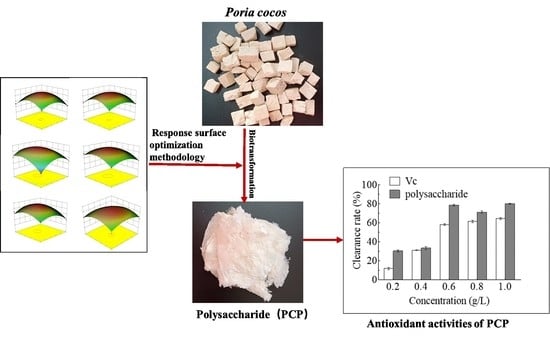
Optimization of Bioprocess Extraction of Poria cocos Polysaccharide (PCP) with Aspergillus niger β-Glucanase and the Evaluation of PCP Antioxidant Property
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation of A. niger HS-5 β-Glucanase
2.1.1. Organism and Culture Conditions
2.1.2. Enzyme Assay
2.2. Effects of Four Single Factors on Extraction Yield of PCP
2.2.1. Enzymolysis Temperature
2.2.2. pH Value
2.2.3. Enzymolysis Time
2.2.4. Enzyme Dosage
2.3. RSM Model Building and Statistical Analysis
0.73X2X3 − 1.86X2X4 − 0.32X3X4 − 0.98X12 − 1.76X22 − 2.46X32 − 1.68X42
2.4. Model Validation
2.5. Antioxidant Activity of PCP In Vitro
2.5.1. Reducing Power
2.5.2. DPPH Radical Scavenging Activity
2.5.3. Superoxide Anion Radical Scavenging Activity
2.5.4. Hydroxyl Radical Scavenging Activity
3. Materials and Methods
3.1. Materials and Reagents
3.2. Single Factor Experiment
3.3. Determination of PCP Yield
3.4. Response Surface Optimization Experiment
3.5. Verification Experiment
3.6. Preparation of Crude PCP
3.7. Antioxidant Property of PCP
3.7.1. Reducing Power
3.7.2. Scavenging of DPPH Radicals
3.7.3. Scavenge of Superoxide Anion Radicals
3.7.4. Scavenge of Hydroxyl Radical
3.8. Data Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jia, X.; Ma, L.; Li, P.; Chen, M.; He, C. Prospects of Poria cocos polysaccharides: Isolation process, structural features and bioactivities. Trends Food Sci. Technol. 2016, 54, 52–62. [Google Scholar] [CrossRef]
- Sun, Y. Biological activities and potential health benefits of polysaccharides from Poria cocos and their derivatives. Int. J. Biol. Macromol. 2014, 68, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Z.; Zhang, J.; Zhao, Y.L.; Li, T.; Shen, T.; Li, J.Q.; Li, W.Y.; Liu, H.G. Mycology, cultivation, traditional uses, phytochemistry and pharmacology of Wolfiporia cocos (Schwein.) Ryvarden et Gilb. J. Ethnopharmacol. 2013, 147, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Ke, R.D.; Lin, S.F.; Chen, Y.; Ji, C.R.; Shu, Q.G. Analysis of chemical composition of polysaccharides from Poria cocos Wolf and its anti-tumor activity by NMR spectroscopy. Carbohydr. Polym. 2010, 80, 31–34. [Google Scholar]
- Zhang, W.X.; Chen, L.; Li, P.; Zhao, J.Z.; Duan, J.Y. Antidepressant and immunosuppressive activities of two polysaccharides from Poria cocos (Schw.) Wolf. Int. J. Biol. Macromol. 2018, 120, 1696–1704. [Google Scholar] [CrossRef]
- Lee, S.; Choi, E.; Yang, S.M.; Ryoo, R.; Moon, E.; Kim, S.H.; Kim, K.H. Bioactive compounds from sclerotia extract of Poria cocos that control adipocyte and osteoblast differentiation. Bioorg. Chem. 2018, 2018. 81, 27–34. [Google Scholar] [CrossRef]
- Nadar, S.S.; Rao, P.; Rathod, V.K. Enzyme assisted extraction of biomolecules as an approach to novel extraction technology: A review. Food Res. Int. 2018, 108, 309–330. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, J.; Zhang, Q.Q.; Zhu, M.H.; Hua, M.L.; Xu, Y.H. Monosaccharide analysis and fingerprinting identification of polysaccharides from Poria cocos and Polyporus umbellatus by HPLC combined with chemometrics methods. Chin. Herb. Med. 2019, 11, 406–411. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, Z.; Mao, J.; Fan, M.; Wu, X. Optimization of ultrasonic-assisted extraction process of Poria cocos polysaccharides by response surface methodology. Carbohyd. Polym. 2009, 77, 713–717. [Google Scholar]
- Yuan, Y.; Hua, H.; Suna, X.H.; Guan, Y.; Chen, C. Rapid determination of polysaccharides and antioxidant activity of Poria cocos using near-infrared spectroscopy combined with chemometrics. Spectrochim. Acta A 2020, 240, 118623. [Google Scholar]
- Chen, X.; Tang, Q.; Chen, Y.; Wang, W.; Li, S. Simultaneous extraction of polysaccharides from Poria cocos by ultrasonic technique and its inhibitory activities against oxidative injury in rats with cervical cancer. Carbohyd. Polym. 2010, 79, 409–413. [Google Scholar] [CrossRef]
- Yang, H.; Wu, Y.; Gan, C.; Yue, T.; Yuan, Y. Characterization and antioxidant activity of a novel polysaccharidefrom Pholidota chinensis Lindl. Antioxidant activity of carboxymethyl (1→3)-β-d-glucan (from the sclerotium of Poria cocos) sulfate (in vitro). Carbohyd. Polym 2016, 138, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, X.; Xu, X.; Zhang, X. Purification, antitumor and antiinflam-mation activities of an alkali-soluble and carboxymethyl polysaccharide CMP33 from Poria cocos. Int. J. Biol. Macrom. 2019, 127, 39–47. [Google Scholar] [CrossRef]
- Pu, Y.; Liu, Z.; Tian, H.; Bao, Y. The immunomodulatory effect of Poria cocos polysaccharides is mediated by the Ca2+/PKC/p38/NF-κB signaling pathway in macrophages. Int. Immunopharmacol. 2019, 72, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Jin, Y.; Zhang, L.; Cheung, P.C.K.; Kennedy, J.F. Structure, molecular size and antitumor activities of polysaccharides from Poria cocos mycelia produced in fermenter. Carbohyd. Polym. 2007, 70, 324–333. [Google Scholar] [CrossRef]
- Sun, S.S.; Wang, K.; Ma, K.; Bao, L.; Liu, H.W. An insoluble polysaccharide from the sclerotium of Poria cocos improves hyperglycemia, hyperlipidemia and hepatic steatosis in ob/ob mice via modulation of gut microbiota. Chin. J. Nat. Med. 2019, 17, 3–14. [Google Scholar] [CrossRef]
- Wu, K.; Fan, J.; Huang, X.; Wu, X.; Guo, C. Hepatoprotective effects exerted by Poria Cocos polysaccharides against acetaminophen-induced liver injury in mice. Int. J. Biol. Macromol. 2018, 114, 137–142. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, S.; Yang, Z.; Zhu, Y.; Wu, Y.; Huang, J.; Mao, J. Oxidation of β-glucan extracted from Poria Cocos and its physiological activities. Carbohyd. Polym. 2011, 85, 798–802. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, Y.; Wang, X.; Huang, X.; Fei, Y.; Yu, Y.; Shou, D. Antioxidant property of water-soluble polysaccharides from Poria cocos Wolf using different extraction methods. Int. J. Biol. Macromol. 2016, 83, 103–110. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, X.; Zhang, J. Optimization of enzyme assisted extraction of polysaccharides from Astragalus membranaceus. Carbohyd. Polym. 2014, 111, 567–575. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, C.P.; Zhai, X.C.; Zhang, Y.; Duan, Z.; Sun, J.R. Optimization of enzyme assisted extraction of polysaccharides from pomegranate peel by response surface methodology and their anti-oxidant potential. Chin. Herb. Med. 2018, 10, 416–423. [Google Scholar] [CrossRef]
- Bian, C.; Xie, N.; Chen, F. Preparation of bioactive water-soluble pachyman hydrolyzed from sclerotial polysaccharides of Poria cocos by hydrolase. Polym. J. 2010, 42, 256–260. [Google Scholar] [CrossRef] [Green Version]
- Yin, X.; You, Q.; Jiang, Z. Optimization of enzyme assisted extraction of polysaccharides from Tricholoma matsutake by response surface methodology. Carbohyd. Polym. 2011, 86, 1358–1364. [Google Scholar] [CrossRef]
- Gao, M.J.; Liu, L.P.; Li, S.; Lyu, J.L.; Jiang, Y.; Zhu, L.; Zhan, X.B.; Zheng, Y.Y. Multi-stage glucose/pachymaran co-feeding enhanced endo-β-1,3-glucanase production by Trichoderma harzianum via simultaneous increases in cell concentration and inductive effect. Bioproc. Biosyst. Eng. 2020, 43, 1479–1486. [Google Scholar] [CrossRef]
- Sánchez, O.F.; Rodriguez, A.M.; Silva, E.; Caicedo, L.A. Sucrose biotransformation to fructooligosaccharides by Aspergillus sp. N74 free cells. Food Bioprocess Technol. 2010, 3, 662–673. [Google Scholar] [CrossRef]
- Ajila, C.M.; Gassara, F.; Brar, S.K.; Verma, M.; Tyagi, R.D.; Valéro, J.R. Polyphenolic antioxidant mobilization in apple pomace by different methods of solid-state fermentation and evaluation of its antioxidant activity. Food Bioprocess Techol. 2012, 5, 2697–2707. [Google Scholar] [CrossRef]
- Chai, Y.; Kan, L.; Zhao, M. Enzymatic extraction optimization, anti-HBV and antioxidant activities of polysaccharides from Viscum coloratum (Kom.) Nakai. Int. J. Biol. Macromol. 2019, 134, 588–594. [Google Scholar] [CrossRef]
- Ivan, A.L.M.; Campanini, M.Z.; Martinez, R.M.; Ferreira, V.S.; Steffen, V.S.; Vicentini, F.T.M.C.; Vilela, F.M.P.; Martins, F.S.; Zarpelon, A.C.; Cunha, T.M. Pyrrolidine dithiocarbamate inhibits UVB-induced skin inflammation and oxidative stress in hairless mice and exhibits antioxidant activity in vitro. J. Photochem. Photobiol. B Biol. 2014, 138, 124–133. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Yu, S.; Yin, L.; Zhao, M.; Han, Z. Synthesized oversulfated and acetylated derivatives of polysaccharide extracted from Enteromorpha linza and their potential antioxidant activity. J. Ethnopharmacol. 2011, 49, 1012–1015. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, S.; Han, L.; Lian, M.; Wen, Z.; Jiayinaguli, T.; Liu, L.; Sun, R.; Cao, Y. Antioxidant activity of carboxymethyl (1→3)-β-D-glucan (from the sclerotium of Poria cocos) sulfate (in vitro). Int. J. Biol. Macromol. 2014, 69, 229–235. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, M.Y.; Nie, S.P.; Li, C.; Wang, Y.X. Purification, composition analysis and antioxidant activity of a polysaccharide from the fruiting bodies of Ganoderma atrum. Food Chem. 2008, 107, 231–241. [Google Scholar] [CrossRef]
- Siddhuraju, P.; Becker, K. The antioxidant and free radical scavenging activities of processed cowpea (Vigna unguiculata (L.) Walp.) seed extracts. Food Chem. 2007, 101, 10–19. [Google Scholar] [CrossRef]





| Standard Number | X1 Temperature (°C) | X2 pH | X3 Time (min) | X4 Enzyme Dose (mL) | Response Value (%) | |
|---|---|---|---|---|---|---|
| Measured Value | Predictive Value | |||||
| 1 | −1 | 0 | 0 | −1 | 8.19 | 8.61 |
| 2 | −1 | 0 | 0 | 1 | 11.66 | 11.13 |
| 3 | 0 | 0 | 0 | 0 | 12.20 | 12.04 |
| 4 | 0 | 1 | 0 | 1 | 6.55 | 6.83 |
| 5 | 0 | 1 | −1 | 0 | 6.51 | 6.98 |
| 6 | 1 | 0 | 1 | 0 | 8.11 | 7.99 |
| 7 | 0 | 0 | 0 | 0 | 12.68 | 12.04 |
| 8 | 0 | 1 | 1 | 0 | 8.18 | 7.90 |
| 9 | −1 | 1 | 0 | 0 | 9.13 | 9.40 |
| 10 | 0 | 1 | 0 | −1 | 10.37 | 9.61 |
| 11 | −1 | 0 | −1 | 0 | 9.92 | 9.52 |
| 12 | 0 | 0 | 1 | −1 | 6.76 | 7.47 |
| 13 | 0 | 0 | 0 | 0 | 11.45 | 12.04 |
| 14 | 0 | 0 | 0 | 0 | 11.93 | 12.04 |
| 15 | 0 | −1 | 0 | −1 | 7.45 | 6.65 |
| 16 | 1 | 0 | 0 | 1 | 9.10 | 8.57 |
| 17 | 0 | 0 | 0 | 0 | 11.93 | 12.04 |
| 18 | 1 | −1 | 0 | 0 | 8.81 | 9.18 |
| 19 | 1 | 0 | 0 | −1 | 8.79 | 9.21 |
| 20 | 0 | 0 | −1 | −1 | 7.37 | 7.38 |
| 21 | 0 | −1 | 1 | 0 | 7.78 | 7.20 |
| 22 | 0 | 0 | −1 | 1 | 9.03 | 8.95 |
| 23 | 0 | −1 | −1 | 0 | 9.04 | 9.21 |
| 24 | 1 | 0 | −1 | 0 | 8.38 | 8.22 |
| 25 | −1 | 0 | 1 | 0 | 9.01 | 8.65 |
| 26 | −1 | −1 | 0 | 0 | 9.58 | 10.19 |
| 27 | 0 | 0 | 1 | 1 | 7.15 | 7.78 |
| 28 | 1 | 1 | 0 | 0 | 8.42 | 8.45 |
| 29 | 0 | −1 | 0 | 1 | 11.08 | 11.32 |
| Source | Sum of Squares | df | Mean Square | F Value | p-Value Prob > F | Significance |
|---|---|---|---|---|---|---|
| Model | 84.95 | 14 | 6.07 | 15.33 | <0.0001 | ** |
| X1-Temperature | 2.88 | 1 | 2.88 | 7.28 | 0.0173 | * |
| X2-pH | 1.75 | 1 | 1.75 | 4.42 | 0.0542 | |
| X3-Time | 0.89 | 1 | 0.89 | 2.24 | 0.1569 | |
| X4-Enzyme dose | 2.65 | 1 | 2.65 | 6.7 | 0.0215 | * |
| X1X2 | 0.9 × 10−3 | 1 | 0.9 × 10−3 | 2.27 × 10−3 | 0.9626 | |
| X1X3 | 0.1 | 1 | 0.1 | 0.26 | 0.619 | |
| X1X4 | 2.5 | 1 | 2.5 | 6.31 | 0.0249 | * |
| X2X3 | 2.15 | 1 | 2.15 | 5.42 | 0.0354 | * |
| X2X4 | 13.88 | 1 | 13.88 | 35.05 | <0.0001 | ** |
| X3X4 | 0.4 | 1 | 0.4 | 1.02 | 0.33 | |
| X12 | 6.23 | 1 | 6.23 | 15.74 | 0.0014 | ** |
| X22 | 19.98 | 1 | 19.98 | 50.48 | <0.0001 | ** |
| X32 | 39.34 | 1 | 39.34 | 99.37 | <0.0001 | ** |
| X42 | 18.31 | 1 | 18.31 | 46.26 | <0.0001 | ** |
| Residual | 5.54 | 14 | 0.4 | |||
| Lack of Fit | 4.73 | 10 | 0.47 | 2.35 | 0.2134 | |
| Pure Error | 0.81 | 4 | 0.2 | |||
| Cor Total | 90.49 | 28 |
| Independent Variables | Levels | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| Enzymolysis temperature (°C) (X1) | 50.00 | 55.00 | 60.00 |
| pH (X2) | 4.50 | 5.00 | 5.50 |
| Enzymolysis time (min) (X3) | 90.00 | 120.00 | 150.00 |
| Enzyme dose (mL) (X4) | 15.00 | 20.00 | 25.00 |
Sample Availability: Samples of the compounds are not available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, P.; Tan, H.; Zhan, J.; Wang, W.; Hu, T.; Li, S. Optimization of Bioprocess Extraction of Poria cocos Polysaccharide (PCP) with Aspergillus niger β-Glucanase and the Evaluation of PCP Antioxidant Property. Molecules 2020, 25, 5930. https://doi.org/10.3390/molecules25245930
Wu P, Tan H, Zhan J, Wang W, Hu T, Li S. Optimization of Bioprocess Extraction of Poria cocos Polysaccharide (PCP) with Aspergillus niger β-Glucanase and the Evaluation of PCP Antioxidant Property. Molecules. 2020; 25(24):5930. https://doi.org/10.3390/molecules25245930
Chicago/Turabian StyleWu, Peng, Hongyuan Tan, Jianfeng Zhan, Weixin Wang, Ting Hu, and Shiming Li. 2020. "Optimization of Bioprocess Extraction of Poria cocos Polysaccharide (PCP) with Aspergillus niger β-Glucanase and the Evaluation of PCP Antioxidant Property" Molecules 25, no. 24: 5930. https://doi.org/10.3390/molecules25245930
APA StyleWu, P., Tan, H., Zhan, J., Wang, W., Hu, T., & Li, S. (2020). Optimization of Bioprocess Extraction of Poria cocos Polysaccharide (PCP) with Aspergillus niger β-Glucanase and the Evaluation of PCP Antioxidant Property. Molecules, 25(24), 5930. https://doi.org/10.3390/molecules25245930