The Influence of Supercritical Carbon Dioxide on Graham Flour Enzyme Polyphenol Oxidase Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Determination of Protein Concentration in Graham Flour
2.2. Protein Concentration after scCO2 Treatment
2.3. PPO Inactivation under scCO2 Treatment
2.4. The Effects of scCO2 on Total Phenolic Contents
2.5. The Effects of scCO2 on Graham Flour Properties
3. Materials and Methods
3.1. Reagents
3.2. Overview of Experimental Procedure
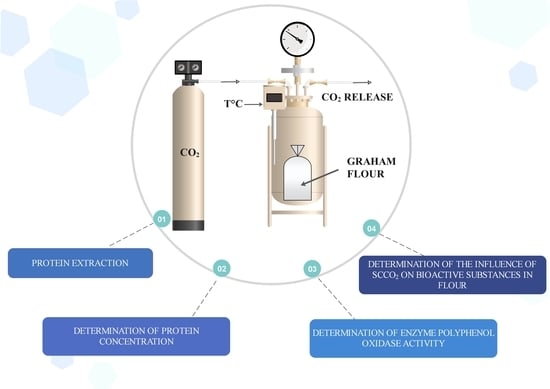
3.3. Supercritical CO2 Treatment
3.4. Optimization of Protein Extraction from Graham Flour
3.5. Determination of Protein Concentration
3.6. Enzyme Assay
3.7. Determination of Total Phenol Content
3.8. Determination of Graham Flour Properties
3.8.1. Particle Size Distribution
3.8.2. Scanning Electron Microscopy of Graham Flour
3.8.3. Moisture Content
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lin, S.; Gao, J.; Jin, X.; Wang, Y.; Dong, Z.; Ying, J.; Zhou, W. Whole-wheat flour particle size influences dough properties, bread structure and in vitro starch digestibility. Food Funct. 2020, 11, 3610–3620. [Google Scholar] [CrossRef]
- Zhai, S.; He, Z.; Wen, W.; Liu, J.; Jin, H.; Yan, J.; Zhang, Y.; Zhang, P.; Wan, Y.; Xia, X. Genetic architecture of polyphenol oxidase activity in wheat flour by genome-wide association study. Crop Sci. 2020, 60, 1281–1293. [Google Scholar] [CrossRef]
- Tinello, F.; Lante, A. Recent advances in controlling polyphenol oxidase activity of fruit and vegetable products. Innov. Food Sci. Emerg. Technol. 2018, 50, 73–83. [Google Scholar] [CrossRef]
- Kuddus, M. Enzymes in Food Technology: Improvements and Innovations; Springer: Singapore, 2018; ISBN 9789811319327. [Google Scholar]
- Nirmal, N.P.; Benjakul, S.; Ahmad, M.; Arfat, Y.A.; Panichayupakaranant, P. Undesirable Enzymatic Browning in Crustaceans: Causative Effects and Its Inhibition by Phenolic Compounds. Crit. Rev. Food Sci. Nutr. 2015, 55, 1992–2003. [Google Scholar] [CrossRef]
- Debelo, H.; Li, M.; Ferruzzi, M.G. Processing Influences on Food Polyphenol Profiles and Biological Activity. Curr. Opin. Food Sci. 2020, 2020. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; Espín, J.C. Phenolic compounds and related enzymes as determinants of quality in fruits and vegetables. J. Sci. Food Agric. 2001, 81, 853–876. [Google Scholar] [CrossRef]
- Nokthai, P.; Lee, V.S.; Shank, L. Molecular Modeling of Peroxidase and Polyphenol Oxidase: Substrate Specificity and Active Site Comparison. Int. J. Mol. Sci. 2010, 11, 3266–3276. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.; Ahmad, K.; Hassan, S.; Imran, M.; Ahmad, N.; Xu, C. Effect of novel technologies on polyphenols during food processing. Innov. Food Sci. Emerg. Technol. 2017, 2018, 361–381. [Google Scholar] [CrossRef]
- Hou, G.G. Asian Noodles: Science, Technology, and Processing, 1st ed.; Wiley: Hoboken, NJ, USA, 2010; ISBN 978-0-470-17922-2. [Google Scholar]
- Zhao, Y.; Huang, Z.-H.; Zhou, H.-M.; Zhu, K.-X.; Guo, X.-N.; Peng, W. Polyphenol oxidase browning in the formation of dark spots on fresh wet noodle sheets: How dark spots formed. Food Chem. 2020, 329, 126800. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.-N.; Wu, S.-H.; Zhu, K.-X. Effect of superheated steam treatment on quality characteristics of whole wheat flour and storage stability of semi-dried whole wheat noodle. Food Chem. 2020, 322, 126738. [Google Scholar] [CrossRef] [PubMed]
- Doble, M.; Kruthiventi, A.K. CHAPTER 9-Industrial Examples. In Green Chemistry and Engineering; Doble, M., Kruthiventi, A.K., Eds.; Academic Press: Burlington, ON, Canada, 2007; pp. 245–296. ISBN 978-0-12-372532-5. [Google Scholar]
- Manzocco, L.; Plazzotta, S.; Spilimbergo, S.; Nicoli, M.C. Impact of high-pressure carbon dioxide on polyphenoloxidase activity and stability of fresh apple juice. LWT-Food Sci. Technol. 2017, 85, 363–371. [Google Scholar] [CrossRef]
- Hojnik Podrepšek, G.; Knez, Ž.; Leitgeb, M. Activation of cellulase cross-linked enzyme aggregates (CLEAs) in scCO2. J. Supercrit. Fluids 2019, 154, 104629. [Google Scholar] [CrossRef]
- Ferrentino, G.; Spilimbergo, S. High pressure carbon dioxide pasteurization of solid foods: Current knowledge and future outlooks. Trends Food Sci. Technol. 2011, 22, 427–441. [Google Scholar] [CrossRef]
- Zhong, Q.; Jin, M. Enhanced Functionalities of Whey Proteins Treated with Supercritical Carbon Dioxide. J. Dairy Sci. 2008, 2008, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, T. Recent progress in biocatalysis using supercritical carbon dioxide. J. Biosci. Bioeng. 2013, 115, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Benito-Román, Ó.; Sanz, M.T.; Illera, A.E.; Melgosa, R.; Benito, J.M.; Beltrán, S. Pectin methylesterase inactivation by High Pressure Carbon Dioxide (HPCD). J. Supercrit. Fluids 2019, 145, 111–121. [Google Scholar] [CrossRef]
- Goyeneche, R.; Di Scala, K.; Roura, S. Biochemical characterization and thermal inactivation of polyphenol oxidase from radish (Raphanus sativus var. sativus). LWT 2013, 54, 57–62. [Google Scholar] [CrossRef]
- Garcia-Palazon, A.; Suthanthangjai, W.; Kajda, P. The effects of high hydrostatic pressure on β-glucosidase, peroxidase and polyphenoloxidase in red raspberry (Rubus idaeus) and strawberry (Fragaria × ananassa). Food Chem. 2004, 88, 7–10. [Google Scholar] [CrossRef]
- Manzocco, L.; Ignat, A.; Valoppi, F.; Burrafato, K.R.; Lippe, G.; Spilimbergo, S.; Nicoli, M.C. Inactivation of mushroom polyphenoloxidase in model systems exposed to high-pressure carbon dioxide. J. Supercrit. Fluids 2016, 107, 669–675. [Google Scholar] [CrossRef]
- Liu, X.; Gao, Y.; Xu, H.; Hao, Q.; Liu, G.; Wang, Q. Inactivation of peroxidase and polyphenol oxidase in red beet (Beta vulgaris L.) extract with continuous high pressure carbon dioxide. Food Chem. 2010, 119, 108–113. [Google Scholar] [CrossRef]
- Chakraborty, S.; Rao, P.S.; Mishra, H.N. Kinetic modeling of polyphenoloxidase and peroxidase inactivation in pineapple (Ananas comosus L.) puree during high-pressure and thermal treatments. Innov. Food Sci. Emerg. Technol. 2015, 27, 57–68. [Google Scholar] [CrossRef]
- Iqbal, A.; Murtaza, A.; Muhammad, Z.; Elkhedir, A.E.; Tao, M.; Xu, X. Inactivation, Aggregation and Conformational Changes of Polyphenol Oxidase from Quince (Cydonia oblonga Miller) Juice Subjected to Thermal and High-Pressure Carbon Dioxide Treatment. Molecules 2018, 23, 1743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- İçi˙er, F.; Yildiz, H.; Baysal, T. Polyphenoloxidase deactivation kinetics during ohmic heating of grape juice. J. Food Eng. 2008, 85, 410–417. [Google Scholar] [CrossRef]
- Cao, X.; Cai, C.; Wang, Y.; Zheng, X. The inactivation kinetics of polyphenol oxidase and peroxidase in bayberry juice during thermal and ultrasound treatments. Innov. Food Sci. Emerg. Technol. 2017, 2018, 169–178. [Google Scholar] [CrossRef]
- Hu, W.; Zhang, Y.; Wang, Y.; Zhou, L.; Leng, X.; Liao, X.; Hu, X. Aggregation and Homogenization, Surface Charge and Structural Change, and Inactivation of Mushroom Tyrosinase in an Aqueous System by Subcritical/Supercritical Carbon Dioxide. Langmuir ACS J. Surf. Colloids 2011, 2011, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Yadav, D.; Patki, P.; Sharma, G.K.; Bawa, A. Effect of microwave heating of wheat grains on the browning of dough and quality of chapattis. Int. J. Food Sci. Technol. 2007, 43, 1217–1225. [Google Scholar] [CrossRef]
- Kushnirov, V.V. Rapid and reliable protein extraction from yeast. Yeast 2000, 16, 857–860. [Google Scholar] [CrossRef]
- Lu, Y.; Chae, M.; Vasanthan, T.; Bressler, D.C. The potential of fiber-depleted starch concentrate produced through air currents assisted particle separation of barley flour in bio-ethanol production. Bioresour. Technol. 2020, 303, 122942. [Google Scholar] [CrossRef]
- Liao, H.; Zhong, K.; Hu, X.; Liao, X. Effect of high pressure carbon dioxide on alkaline phosphatase activity and quality characteristics of raw bovine milk. Innov. Food Sci. Emerg. Technol. 2019, 52, 457–462. [Google Scholar] [CrossRef]
- Kamat, S.V.; Beckman, E.J.; Russell, A.J. Enzyme Activity in Supercritical Fluids. Crit. Rev. Biotechnol. 1995, 15, 41–71. [Google Scholar] [CrossRef]
- Marszałek, K.; Doesburg, P.; Starzonek, S.; Szczepańska, J.; Woźniak, Ł.; Lorenzo, J.M.; Skąpska, S.; Rzoska, S.; Barba, F.J. Comparative effect of supercritical carbon dioxide and high pressure processing on structural changes and activity loss of oxidoreductive enzymes. J. CO2 Util. 2019, 29, 46–56. [Google Scholar] [CrossRef]
- Marszałek, K.; Krzyżanowska, J.; Woźniak, Ł.; Skąpska, S. Kinetic modelling of polyphenol oxidase, peroxidase, pectin esterase, polygalacturonase, degradation of the main pigments and polyphenols in beetroot juice during high pressure carbon dioxide treatment. LWT-Food Sci. Technol. 2017, 85, 412–417. [Google Scholar] [CrossRef]
- Solaesa, Á.G.; Villanueva, M.; Beltrán, S.; Ronda, F. Characterization of Quinoa Defatted by Supercritical Carbon Dioxide. Starch Enzymatic Susceptibility and Structural, Pasting and Thermal Properties. Food Bioprocess Technol. 2019, 12, 1593–1602. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.-W.; Rahman, M.S.; Kim, A.-N.; Lee, K.-Y.; Park, C.-Y.; Kerr, W.L.; Choi, S.-G. Comparative study of the quality characteristics of defatted soy flour treated by supercritical carbon dioxide and organic solvent. J. Food Sci. Technol. 2017, 54, 2485–2493. [Google Scholar] [CrossRef]
- Hironaka, K.; Ishibashi, K.-I.; Koaze, H.; Shirasaka, H.; Matsuda, K.; Sato, T.; Kojima, M.; Mori, M.; Tsuda, S.; Takada, A. Effects of polyphenol content, polyphenol oxidase activity and pH on blackspot bruise of Japanese potato varieties. Food Preserv. Sci. 2006, 32, 67–72. [Google Scholar] [CrossRef] [Green Version]
- Esmaeili, N.; Ebrahimzadeh, H.; Abdi, K. Correlation between polyphenol oxidase (PPO) activity and total phenolic contents in Crocus sativus L. corms during dormancy and sprouting stages. Pharmacogn. Mag. 2017, 13, 519. [Google Scholar] [CrossRef]
- Brown, Z.K. The Drying of Foods Using Supercritical Carbon Dioxide; University of Birmingham: Birmingham, UK, 2010. [Google Scholar]
- Lapčíková, B.; Burešová, I.; Lapčík, L.; Dabash, V.; Valenta, T. Impact of particle size on wheat dough and bread characteristics. Food Chem. 2019, 297, 124938. [Google Scholar] [CrossRef]
- Primožič, M.; Čolnik, M.; Knez, Ž.; Leitgeb, M. Advantages and disadvantages of using SC CO2 for enzyme release from halophilic fungi. J. Supercrit. Fluids 2019, 143, 286–293. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Behzadi, R.; Sadeghizadeh, M.; Movahedi, A.A.M.; Sabouri, A.A.; Sattari, T.N. Gain of human tyrosinase DOPA oxidase activity in artificial M374 Asp mutant. Life Sci. J. 2013, 10, 194–198. [Google Scholar]
- Škerget, M.; Kotnik, P.; Hadolin, M.; Hraš, A.R.; Simonič, M.; Knez, Ž. Phenols, proanthocyanidins, flavones and flavonols in some plant materials and their antioxidant activities. Food Chem. 2005, 89, 191–198. [Google Scholar] [CrossRef]







| Sample | Mean Particle Size (µm) | Moisture Content (%) |
|---|---|---|
| Untreated graham flour | 82 ± 5 | 12.4 ± 1.6 |
| scCO2-treated graham flour | 74 ± 5 | 11.9 ± 1.7 |
| Sample Abbreviation | Conditions | Centrifugation (rpm) |
|---|---|---|
| GM8000 | Graham flour stored at room temperature | 8000 |
| GHL8000 | Graham flour stored in a refrigerator (2–8 °C) | 8000 |
| GST8000 | Graham flour stored at room temperature with the addition of glass beads | 8000 |
Sample Availability: Samples of the compounds are not available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hojnik Podrepšek, G.; Knez, Ž.; Leitgeb, M. The Influence of Supercritical Carbon Dioxide on Graham Flour Enzyme Polyphenol Oxidase Activity. Molecules 2020, 25, 5981. https://doi.org/10.3390/molecules25245981
Hojnik Podrepšek G, Knez Ž, Leitgeb M. The Influence of Supercritical Carbon Dioxide on Graham Flour Enzyme Polyphenol Oxidase Activity. Molecules. 2020; 25(24):5981. https://doi.org/10.3390/molecules25245981
Chicago/Turabian StyleHojnik Podrepšek, Gordana, Željko Knez, and Maja Leitgeb. 2020. "The Influence of Supercritical Carbon Dioxide on Graham Flour Enzyme Polyphenol Oxidase Activity" Molecules 25, no. 24: 5981. https://doi.org/10.3390/molecules25245981
APA StyleHojnik Podrepšek, G., Knez, Ž., & Leitgeb, M. (2020). The Influence of Supercritical Carbon Dioxide on Graham Flour Enzyme Polyphenol Oxidase Activity. Molecules, 25(24), 5981. https://doi.org/10.3390/molecules25245981