Deep Oxidative Desulfurization of Fuels in the Presence of Brönsted Acidic Polyoxometalate-Based Ionic Liquids
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of the Catalysts
- Calc. for Py-1: C, 20.84; H, 1.99; N, 3.47. Found: C, 20.87; H, 1.98; N, 3.53.
- Calc. for Py-2: C, 23.02; H, 2.4; N, 3.36. Found: C, 22.95; H, 2.35; N, 3.38.
- Calc. for Py-3: C, 28.76; H, 3.49; N, 3.05. Found: C, 27.96; H, 3.27; N, 3.0.
- Calc. for NK-1: C, 26.97; H, 3.15; N, 3.15. Found: C, 26.69; H, 3.09; N, 3.17.
- Calc. for NK-2: C, 21.78; H, 2.61; N, 2.54. Found: C, 20.97; H, 2.13; N, 2.27.
- Calc. for NK-3: C, 10.84; H, 1.49; N, 1.26. Found: C, 10.97; H, 1.63; N, 1.57.
- Calc. for NK-4: C, 38.78; H, 5.39; N, 2.51. Found: C, 39.94; H, 5.99; N, 2.37.
2.2. Spectral Characterizations
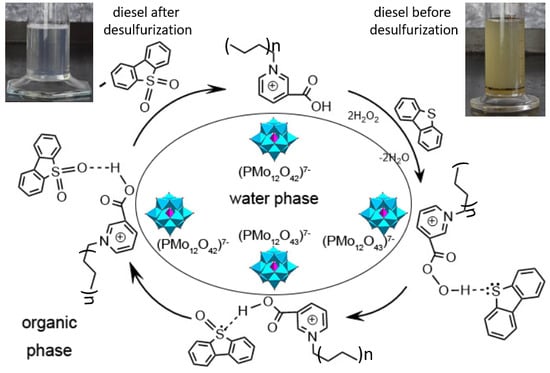
2.3. Catalytic Tests
- -
- the formation of peroxocomplexes by anions, which are intended for the oxidation of organosulfur compounds;
- -
- the formation of the corresponding peracid, also capable of oxidizing organosulfur compounds;
- -
- Bronsted acid site for activation of sulfur atoms;
- -
- a cation playing the role of an interphase carrier due to the ability to control hydrophobic properties by varying the length of the alkyl chain.
3. Experimental
3.1. Materials
3.2. Synthesis of Ionic Liquids
3.3. Catalyst Characterization
3.4. Catalytic Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhao, H.; Baker, G.A. Oxidative desulfurization of fuels using ionic liquids: A review. Front. Chem. Sci. Eng. 2015, 9, 262–279. [Google Scholar] [CrossRef]
- Bhutto, A.W.; Abro, R.; Gao, S.R.; Abbas, T.; Chen, X.C.; Yu, G.R. Oxidative desulfurization of fuel oils using ionic liquids: A review. J. Taiwan Inst. Chem. Eng. 2016, 62, 84–97. [Google Scholar] [CrossRef]
- Babich, I.V.; Moulijn, J.A. Science and technology of novel processes for deep desulfurization of oil refinery streams: A review. Fuel 2003, 82, 607–631. [Google Scholar] [CrossRef]
- Houda, S.; Lancelot, C.; Blanchard, P.; Poinel, L.; Lamonier, C. Oxidative Desulfurization of Heavy Oils with High Sulfur Content: A Review. Catalysts 2018, 8, 344. [Google Scholar] [CrossRef] [Green Version]
- Akopyan, A.V.; Fedorov, R.A.; Andreev, B.V.; Tarakanova, A.V.; Anisimov, A.V.; Karakhanov, E.A. Oxidative Desulfurization of Hydrocarbon Feedstock. Russ. J. Appl. Chem. 2018, 91, 529–542. [Google Scholar] [CrossRef]
- Qiu, L.; Cheng, Y.; Yang, C.P.; Zeng, G.M.; Long, Z.Y.; Wei, S.N.; Zhao, K.; Luo, L. Oxidative desulfurization of dibenzothiophene using a catalyst of molybdenum supported on modified medicinal stone. Rsc Adv. 2016, 6, 17036–17045. [Google Scholar] [CrossRef]
- Mokhtar, W.N.A.W.; Abu Bakar, W.A.W.; Ali, R.; Kadir, A.A.A. Development of bimetallic and trimetallic oxides doped on molybdenum oxide based material on oxidative desulfurization of diesel. Arab. J. Chem. 2018, 11, 1201–1208. [Google Scholar] [CrossRef] [Green Version]
- Campos-Martin, J.M.; Capel-Sanchez, M.C.; Perez-Presas, P.; Fierro, J.L.G. Oxidative processes of desulfurization of liquid fuels. J. Chem. Technol. Biotechnol. 2010, 85, 879–890. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Z.X.; Lue, H.Y.; Zhang, Y.N.; Li, C. Oxidative desulfurization of fuel oils. Chin. J. Catal. 2011, 32, 707–715. [Google Scholar] [CrossRef]
- Polikarpova, P.; Akopyan, A.; Shigapova, A.; Glotov, A.; Anisimov, A.; Karakhanov, E. Oxidative Desulfurization of Fuels Using Heterogeneous Catalysts Based on MCM-41. Energy Fuels 2018, 32, 10898–10903. [Google Scholar] [CrossRef]
- Subhan, S.; Rahman, A.U.; Yaseen, M.; Rashid, H.U.; Ishaq, M.; Sahibzada, M.; Tong, Z.F. Ultra-fast and highly efficient catalytic oxidative desulfurization of dibenzothiophene at ambient temperature over low Mn loaded Co-Mo/Al2O3 and Ni-Mo/Al2O3 catalysts using NaClO as oxidant. Fuel 2019, 237, 793–805. [Google Scholar] [CrossRef]
- Campos-Martin, J.M.; Blanco-Brieva, G.; Fierro, J.L.G. Hydrogen peroxide synthesis: An outlook beyond the anthraquinone process. Angew. Chem. -Int. Ed. 2006, 45, 6962–6984. [Google Scholar] [CrossRef] [PubMed]
- Al-Shahrani, F.; Xiao, T.C.; Llewellyn, S.A.; Barri, S.; Jiang, Z.; Shi, H.H.; Martinie, G.; Green, M.L.H. Desulfurization of diesel via the H2O2 oxidation of aromatic sulfides to sulfones using a tungstate catalyst. Appl. Catal. B-Environ. 2007, 73, 311–316. [Google Scholar] [CrossRef]
- Martínez-Vargas, D.X.; De La Rosa, J.R.; Sandoval-Rangel, L.; Guzmán-Mar, J.L.; Garza-Navarro, M.A.; Lucio-Ortiz, C.J.; De Haro-Del Río, D.A. 5-Hydroxymethylfurfural catalytic oxidation under mild conditions by Co (II), Fe (III) and Cu (II) Salen complexes supported on SBA-15: Synthesis, characterization and activity. Appl. Catal. Ageneral 2017, 547, 132–145. [Google Scholar] [CrossRef]
- Li, J.-K.; Xu, Y.-Q.; Hu, C.-W. In situ synthesis of a novel dioxidovanadium-based nickel complex as catalyst for deep oxidative desulfurization with molecular oxygen. Inorg. Chem. Commun. 2015, 60, 12–14. [Google Scholar] [CrossRef]
- Julião, D.; Gomes, A.C.; Cunha-Silva, L.; Pillinger, M.; Lopes, A.D.; Valença, R.; Ribeiro, J.C.; Gonçalves, I.S.; Balula, S.S. Dichlorodioxomolybdenum(VI) complexes bearing oxygen-donor ligands as catalysts for oxidative desulfurization of simulated and real diesel. Catal. Commun. 2019, 128, 105704–105705. [Google Scholar] [CrossRef]
- Komintarachat, C.; Trakarnpruk, W. Oxidative desulfurization using polyoxometalates. Ind. Eng. Chem. Res. 2006, 45, 1853–1856. [Google Scholar] [CrossRef]
- Rakhmanov, E.V.; Tarakanova, A.V.; Valieva, T.; Akopyan, A.V.; Litvinova, V.V.; Maksimov, A.L.; Anisimov, A.V.; Vakarin, S.V.; Semerikova, O.L.; Zaikov, Y.P. Oxidative desulfurization of diesel fraction with hydrogen peroxide in the presence of catalysts based on transition metals. Pet. Chem. 2014, 54, 48–50. [Google Scholar] [CrossRef]
- Rafiee, E.; Rezaei, S. Deep extractive desulfurization and denitrogenation of various model oils by H3+nPMo12-nVnO40 supported on silica-encapsulated gamma-Fe2O3 nanoparticles for industrial effluents applications. J. Taiwan Inst. Chem. Eng. 2016, 61, 174–180. [Google Scholar] [CrossRef]
- Jiang, X.; Li, H.M.; Zhu, W.S.; He, L.N.; Shu, H.M.; Lu, J.D. Deep desulfurization of fuels catalyzed by surfactant-type decatungstates using H2O2 as oxidant. Fuel 2009, 88, 431–436. [Google Scholar] [CrossRef]
- Lu, H.; Ren, W.; Wang, H.; Wang, Y.; Chen, W.; Suo, Z. Deep desulfurization of diesel by ionic liquid extraction coupled with catalytic oxidation using an Anderson-type catalyst [(C4H9)4N]4NiMo6O24H6. Appl. Catal. A-Gen. 2013, 453, 376–382. [Google Scholar] [CrossRef]
- Ibrahim, M.H.; Hayyan, M.; Hashim, M.A.; Hayyan, A. The role of ionic liquids in desulfurization of fuels: A review. Renew. Sustain. Energy Rev. 2017, 76, 1534–1549. [Google Scholar] [CrossRef]
- Akopyan, A.V.; Eseva, E.A.; Polikarpova, P.D.; Baigil’diev, T.M.; Rodin, I.A.; Anishnov, A.V. Catalytic Activity of Polyfunctional Ionic Liquids in Oxidation of Model Sulfur Organic Compounds. Russ. J. Appl. Chem. 2019, 92, 569–575. [Google Scholar] [CrossRef]
- Hao, L.; Sun, L.; Su, T.; Hao, D.; Liao, W.; Deng, C.; Ren, W.; Zhang, Y.; Lü, H. Polyoxometalate-based ionic liquid catalyst with unprecedented activity and selectivity for oxidative desulfurization of diesel in [Omim]BF4. Chem. Eng. J. 2019, 358, 419–426. [Google Scholar] [CrossRef]
- Lu, L.; Cheng, S.; Gao, J.; Gao, G.; He, M. Deep oxidative desulfurization of fuels catalyzed by ionic liquid in the presence of H2O2. Energy Fuels 2007, 21, 383–384. [Google Scholar] [CrossRef]
- Jiang, W.; Zhu, W. Mechanism and optimization for oxidative desulfurization of fuels catalyzed by Fenton-like catalysts in hydrophobic ionic liquid. J. Mol. Catal. A: Chem. 2014, 382, 8–14. [Google Scholar] [CrossRef]
- Li, H.; He, L.; Lu, J.; Zhu, W.; Jiang, X.; Wang, Y.; Yan, Y. Deep oxidative desulfurization of fuels catalyzed by phosphotungstic acid in ionic liquids at room temperature. Energy Fuels 2009, 23, 1354–1357. [Google Scholar] [CrossRef]
- Yazu, K.; Makino, M.; Ukegawa, K. Oxidative desulfurization of diesel oil with hydrogen peroxide in the presence of acid catalyst in diesel oil/acetic acid biphasic system. Chem. Lett. 2004, 33, 1306–1307. [Google Scholar] [CrossRef]
- Zhu, Y.F.; Zhu, M.Y.; Kang, L.H.; Yu, F.; Dai, B. Phosphotungstic acid supported on mesoporous graphitic carbon nitride as catalyst for oxidative desulfurization of fuel. Ind. Eng. Chem. Res. 2015, 54, 2040–2047. [Google Scholar] [CrossRef]
- Pham, X.N.; Tran, D.L.; Pham, T.D.; Nguyen, Q.M.; Thi, V.T.T.; Van, H.D. One-step synthesis, characterization and oxidative desulfurization of 12-tungstophosphoric heteropolyanions immobilized on amino functionalized SBA-15. Adv. Powder Technol. 2018, 29, 58–65. [Google Scholar] [CrossRef] [Green Version]
- Krivtsov, E.B.; Golovko, A.K. The kinetics of oxidative desulfurization of diesel fraction with a hydrogen peroxide-formic acid mixture. Pet. Chem. 2014, 54, 51–57. [Google Scholar] [CrossRef]
- Liu, D.; Gui, J.; Song, L.; Zhang, X.; Sun, Z. Deep desulfurization of diesel fuel by extraction with task-specific ionic liquids. Pet. Sci. Technol. 2008, 26, 973–982. [Google Scholar] [CrossRef]
- Gui, J.Z.; Liu, D.; Sun, Z.L.; Liu, D.S.; Min, D.; Song, B.; Peng, X.L. Deep oxidative desulfurization with task-specific ionic liquids: An experimental and computational study. J. Mol. Catal. A-Chem. 2010, 331, 64–70. [Google Scholar] [CrossRef]
- Zhu, W.S.; Li, H.M.; Jiang, X.; Yan, Y.S.; Lu, J.D.; He, L.N.; Xia, J.X. Commercially available molybdic compound-catalyzed ultra-deep desulfurization of fuels in ionic liquids. Green Chem. 2008, 10, 641–646. [Google Scholar] [CrossRef]
- Kulkarni, P.S.; Afonso, C.A.M. Deep desulfurization of diesel fuel using ionic liquids: Current status and future challenges. Green Chem. 2010, 12, 1139–1149. [Google Scholar] [CrossRef]
- RocchiccioliDeltcheff, C.; Aouissi, A.; Bettahar, M.; Launay, S.; Fournier, M. Catalysis by 12-molybdophosphates: 1. Catalytic reactivity of 12-molybdophosphoric acid related to its thermal behavior investigated through IR, Raman, polarographic, and X-ray diffraction studies: A comparison with 12-molybdosilicic acid. J. Catal. 1996, 164, 16–27. [Google Scholar] [CrossRef]
- Torres-García, E.; Galano, A.; Rodriguez-Gattorno, G. Oxidative desulfurization (ODS) of organosulfur compounds catalyzed by peroxo-metallate complexes of WOx–ZrO2: Thermochemical, structural, and reactivity indexes analyses. J. Catal. 2011, 282, 201–208. [Google Scholar] [CrossRef]
- Thorsteinsson, T.; Masson, M.; Kristinsson, K.G.; Hjalmarsdottir, M.A.; Hilmarsson, H.; Loftsson, T. Soft antimicrobial agents: Synthesis and activity of labile environmentally friendly long chain quaternary ammonium compounds. J. Med. Chem. 2003, 46, 4173–4181. [Google Scholar] [CrossRef]
- Chevalier, A.; Zhang, Y.M.; Khdour, O.M.; Hecht, S.M. Selective Functionalization of Antimycin A Through an N-Transacylation Reaction. Org. Lett. 2016, 18, 2395–2398. [Google Scholar] [CrossRef] [Green Version]
- Zhu, W.S.; Huang, W.L.; Li, H.M.; Zhang, M.; Jiang, W.; Chen, G.Y.; Han, C.R. Polyoxometalate-based ionic liquids as catalysts for deep desulfurization of fuels. Fuel Process. Technol. 2011, 92, 1842–1848. [Google Scholar] [CrossRef]
- Chang, J.C.; Yang, C.H.; Yang, H.H.; Hsueh, M.L.; Ho, W.Y.; Chang, J.Y.; Sun, I.W. Pyridinium molten salts as co-adsorbents in dye-sensitized solar cells. Sol. Energy 2011, 85, 174–179. [Google Scholar] [CrossRef]
- Ford, L.; Ylijoki, K.E.O.; Garcia, M.T.; Singer, R.D.; Scammells, P.J. Nitrogen-Containing Ionic Liquids: Biodegradation Studies and Utility in Base-Mediated Reactions. Aust. J. Chem. 2015, 68, 849–857. [Google Scholar] [CrossRef] [Green Version]
- ASTM International. ASTM D4294-10, Standard Test Method for Sulfur in Petroleum and Petroleum Products by Energy Dispersive Xray Fluorescence Spectrometry; ASTM International: West Conshohocken, PA, USA, 2010. [Google Scholar]
Sample Availability: Samples of the compounds NK-1, NK-2, NK-3, NK-4, Py-1, Py-2, Py-3 are available from the authors. |











| Ionic Liquid | Designation | Calculated Values, % Mass. | Found Amount, % Mass. | ||
|---|---|---|---|---|---|
| Mo | P | Mo | P | ||
| (NKBu)7PMo12O42 | NK – 1 | 36.98 | 0.99 | 37.44 ± 0.31 | 1.29 ± 0.1 |
| (NKBu)5H2PMo12O42 | NK – 2 | 41.81 | 1.12 | 41.48 ± 0.3 | 1.45 ± 0.11 |
| (NKBu)2H5PMo12O42 | NK – 3 | 52.01 | 1.40 | 51.04 ± 0.27 | 1.54 ± 0.13 |
| (NKC12)7PMo12O42 | NK – 4 | 29.55 | 0.81 | 27.61 ± 0.3 | 1.24 ± 0.08 |
| (PyAc)7PMo12O42 | Py – 1 | 40.84 | 1.10 | 40.64 ± 0.31 | 1.27 ± 0.11 |
| (PyPr)7PMo12O42 | Py – 2 | 39.47 | 1.06 | 37.57 ± 0.31 | 1.20 ± 0.09 |
| (PyHex)7PMo12O42 | Py – 3 | 35.85 | 0.96 | 37.40 ± 0.31 | 1.08 ± 0.1 |
| Substrate | Conversion, % |
|---|---|
| methylphenylsulfide | 100 |
| dibenzylsulfide | 100 |
| benzothiophene | 51 |
| 5-methylbenzothiophene | 25 |
| Dibenzothiophene | 71 |
| 4-methyldibenzothiophene | 96 |
| 4,6-dimethyldibenzothiophene | 64 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akopyan, A.; Eseva, E.; Polikarpova, P.; Kedalo, A.; Vutolkina, A.; Glotov, A. Deep Oxidative Desulfurization of Fuels in the Presence of Brönsted Acidic Polyoxometalate-Based Ionic Liquids. Molecules 2020, 25, 536. https://doi.org/10.3390/molecules25030536
Akopyan A, Eseva E, Polikarpova P, Kedalo A, Vutolkina A, Glotov A. Deep Oxidative Desulfurization of Fuels in the Presence of Brönsted Acidic Polyoxometalate-Based Ionic Liquids. Molecules. 2020; 25(3):536. https://doi.org/10.3390/molecules25030536
Chicago/Turabian StyleAkopyan, Argam, Ekaterina Eseva, Polina Polikarpova, Anastasia Kedalo, Anna Vutolkina, and Aleksandr Glotov. 2020. "Deep Oxidative Desulfurization of Fuels in the Presence of Brönsted Acidic Polyoxometalate-Based Ionic Liquids" Molecules 25, no. 3: 536. https://doi.org/10.3390/molecules25030536
APA StyleAkopyan, A., Eseva, E., Polikarpova, P., Kedalo, A., Vutolkina, A., & Glotov, A. (2020). Deep Oxidative Desulfurization of Fuels in the Presence of Brönsted Acidic Polyoxometalate-Based Ionic Liquids. Molecules, 25(3), 536. https://doi.org/10.3390/molecules25030536