Deep Eutectic Solvents as Effective Reaction Media for the Synthesis of 2-Hydroxyphenylbenzimidazole-Based Scaffolds en Route to Donepezil-Like Compounds
Abstract
:1. Introduction
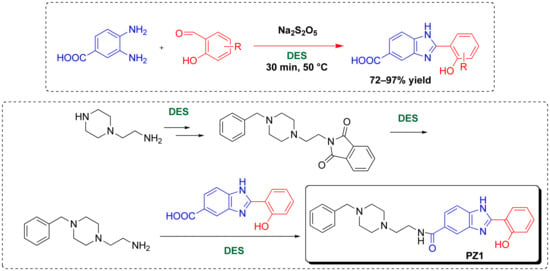
2. Results and Discussion
3. Materials and Methods
3.1. General Methods
3.2. Preparation of Deep Eutectic Solvents (DESs)
3.3. Synthesis and Characterization Data of 2-[2-(4-benzylpiperazin-1-yl)ethyl]isoindoline-1,3-dione (2)
3.4. Synthesis and Characterization Data of 2-(4-benzyl-1-piperazinyl)ethanamine (4)
3.5. Synthesis and Characterization Data of 2-(2-hydroxyphenyl)-1H-benzo[d]imidazole-5-carboxylic Acid (7a)
3.6. Synthesis and Characterization Data of 2-(2-hydroxyphenyl)-1H-benzo[d]imidazole-5-carboxylic Acid Derivatives (7b–h)
3.7. Synthesis and Characterization Data of N-(2-(4-benzylpiperazin-1-yl)ethyl)-2-(2-hydroxy-phenyl)-1H-benzo[d]imidazole-5-carboxamide (PZ1) (8)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cacabelos, R. Have there been improvements in Alzheimer’s disease drug discovery over the past 5 years? Expert Opin. Drug Discov. 2018, 13, 523–538. [Google Scholar] [CrossRef] [PubMed]
- Piemontese, L.; Loiodice, F.; Chaves, S.; Santos, M.A. The Therapy of Alzheimer’s Disease: Towards a New Generation of Drugs. Front. Clin. Drug Res. Alzheimer Disord. 2019, 8, 33–80. [Google Scholar]
- Fiest, K.M.; Roberts, J.I.; Maxwell, C.J.; Hogan, D.B.; Smith, E.E.; Frolkis, A.; Cohen, A.; Kirk, A.; Pearson, D.; Pringsheim, T.; et al. The prevalence and incidence of dementia due to Alzheimer’s disease: A systematic review and meta-analysis. Can. J. Neurol. Sci. 2016, 43, S51–S82. [Google Scholar] [CrossRef] [PubMed]
- GBD 2015. Neurological Disorders Collaborator Group. Global, regional, and national burden of neurological disorders during 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol. 2017, 16, 877–897. [Google Scholar] [CrossRef] [Green Version]
- Cummings, J.; Aisen, P.S.; DuBois, B.; Frölich, L.; Jack, C.R.; Jones, R.W.; Morris, J.C.; Raskin, J.; Dowsett, S.A.; Scheltens, P. Drug development in Alzheimer’s disease: The path to 2025. Alzheimers Res. Ther. 2016, 8, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cummings, J.L.; Morstorf, T.; Zhong, K. Alzheimer’s disease drug-development pipeline: Few candidates, frequent failures. Alzheimers Res. Ther. 2014, 6, 37. [Google Scholar] [CrossRef] [Green Version]
- Cummings, J.; Lee, G.; Mortsdorf, T.; Ritter, A.; Zhong, K. Alzheimer’s disease drug development pipeline: 2019. Alzheimers Dement. 2019, 5, 272–293. [Google Scholar] [CrossRef]
- Dudley, J.; Berliocchi, L. Drug Repositioning: Approaches and Applications for Neurotherapeutic; Taylor&Francis Group, CRC Press: Boca Raton, FL, USA, 2017; ISBN 9781482220834. [Google Scholar]
- Guzior, N.; Wieckowska, A.; Panek, D.; Malawska, B. Recent development of multifunctional agents as potential drug candidates for the treatment of Alzheimer’s disease. Curr. Med. Chem. 2015, 22, 373–404. [Google Scholar] [CrossRef]
- Das, S.; Basu, S. Multi-targeting strategies for alzheimer’s disease therapeutics: Pros and cons. Curr. Top. Med. Chem. 2017, 17, 3017–3061. [Google Scholar] [CrossRef]
- Piemontese, L. New approaches for prevention and treatment of Alzheimer’s disease: A fascinating challenge. Neural Regen. Res. 2017, 12, 405–406. [Google Scholar] [CrossRef]
- Mattson, M.P. Pathways towards and away from Alzheimer’s disease. Nature 2004, 430, 631–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daoud, I.; Melkemi, N.; Salah, T.; Ghalem, S. Combined QSAR, molecular docking and molecular dynamics study on new Acetylcholinesterase and Butyrylcholinesterase inhibitors. Comput. Biol. Chem. 2018, 74, 304–326. [Google Scholar] [CrossRef] [PubMed]
- Darvesh, S.; Hopkins, D.A.; Geula, C. Neurobiology of butyrylcholinesterase. Nat. Rev. Neurosci. 2003, 4, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Greig, N.H.; Lahiri, D.K.; Sambamurti, K.; Kumar, S. Butyrylcholinesterase: An important new target in Alzheimer’s disease therapy. Int. Psychogeriatr. 2002, 14 (Suppl. 1), 77–91. [Google Scholar] [CrossRef]
- Rivera, I.; Capone, R.; Cauvi, D.M.; Arispe, N.; De Maio, A. Modulation of Alzheimer’s amyloid β peptide oligomerization and toxicity by extracellular Hsp70. Cell. Stress Chaperones 2018, 23, 269–279. [Google Scholar] [CrossRef]
- Chaves, S.; Piemontese, L.; Hiremathad, A.; Santos, M.A. Hydroxypyridinone Derivatives: A Fascinating Class of Chelators with Therapeutic Applications–An Update. Curr. Med. Chem. 2018, 25, 97–112. [Google Scholar] [CrossRef]
- Crews, L.; Masliah, E. Molecular mechanisms of neurodegeneration in Alzheimer’s disease. Hum. Mol. Genet. 2010, 19, R12–R20. [Google Scholar] [CrossRef]
- Piemontese, L.; Tomás, D.; Hiremathad, A.; Capriati, V.; Candeias, E.; Cardoso, S.M.; Chaves, S.; Santos, M.A. Donepezil structure-based hybrids as potential multifunctional anti-Alzheimer’s drug candidates. J. Enzyme Inhib. Med. Chem. 2018, 33, 1212–1224. [Google Scholar] [CrossRef] [Green Version]
- Hiremathad, A.; Keri, R.S.; Esteves, A.R.; Cardoso, S.M.; Chaves, S.; Santos, M.A. Novel Tacrine- Hydroxyphenylbenzimidazole hybrids as potential multitarget drug candidates for Alzheimer’s disease. Eur. J. Med. Chem. 2018, 148, 255–267. [Google Scholar] [CrossRef]
- Chaves, S.; Hiremathad, A.; Tomás, D.; Keri, R.S.; Piemontese, L.; Santos, M.A. Exploring the chelating capacity of 2-hydroxyphenyl-benzimidazole based hybrids with multi-target ability as anti-Alzheimer’s agents. New J. Chem. 2018, 42, 16503–16515. [Google Scholar] [CrossRef]
- Salahuddin; Shaharyar, M.; Mazumder, A. Benzimidazoles: A biologically active compounds. Arab. J. Chem. 2017, 10, S157–S173. [Google Scholar]
- Singh, V.K.; Parle, A. The intriguing benzimidazole: A review. Int. J. Pharm. Sci. Res. 2019, 10, 1540–1552. [Google Scholar]
- Singh, P.K.; Silakari, O. Benzimidazole: Journey from Single Targeting to Multitargeting Molecule. In Key Heterocycle Cores for Designing Multitargeting Molecules, 1st ed.; Silakari, O., Ed.; Elsevier Ltd.: Amsterdam, The Netherland, 2018; pp. 31–52. [Google Scholar]
- Gulcan, H.O.; Mavideniz, A.; Sahin, M.F.; Orhan, I.E. Benzimidazole-derived Compounds Designed for Different Targets of Alzheimer’s Disease. Curr. Med. Chem. 2019, 26, 3260–3278. [Google Scholar] [CrossRef] [PubMed]
- Hiremathad, A.; Piemontese, L. Heterocyclic compounds as key structures for the interaction with old and new targets in Alzheimer’s disease therapy. Neural Regen. Res. 2017, 12, 1256–1261. [Google Scholar] [PubMed]
- Piemontese, L.; Vitucci, G.; Catto, M.; Laghezza, A.; Perna, F.M.; Rullo, M.; Loiodice, F.; Capriati, V.; Solfrizzo, M. Natural Scaffolds with Multi-Target Activity for the Potential Treatment of Alzheimer’s Disease. Molecules 2018, 23, 2182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deep Eutectic Solvents: Synthesis, Properties, and Applications, 1st ed.; Ramón, D.J.; Guillena, G. (Eds.) Wiley-VCH: Weinheim, Germany, 2019; pp. 1–384. [Google Scholar]
- Alonso, D.A.; Baeza, A.; Chinchilla, R.; Guillena, G.; Pastor, I.M.; Ramón, D.J. Deep Eutectic Solvents: The Organic Reaction Medium of the Century. Eur. J. Org. Chem. 2016, 612–632. [Google Scholar] [CrossRef] [Green Version]
- Perna, F.M.; Vitale, P.; Capriati, V. Deep eutectic solvents and their applications as green solvents. Curr. Opin. Green Sust. Chem. 2020, 21, 27–33. [Google Scholar] [CrossRef]
- Paradiso, V.M.; Clemente, A.; Summo, C.; Pasqualone, A.; Caponio, F. Towards green analysis of virgin olive oil phenolic compounds: Extraction by a natural deep eutectic solvent and direct spectrophotometric detection. Food Chem. 2016, 212, 43–47. [Google Scholar] [CrossRef]
- Piemontese, L.; Perna, F.M.; Logrieco, A.; Capriati, V.; Solfrizzo, M. Deep Eutectic Solvents as Novel and Effective Extraction Media for Quantitative Determination of Ochratoxin A in Wheat and Derived Products. Molecules 2017, 22, 121. [Google Scholar] [CrossRef] [Green Version]
- Pena-Pereira, F.; Namieśnik, J. Ionic liquids and deep eutectic mixtures: Sustainable solvents for extraction processes. ChemSusChem 2014, 7, 1784–1800. [Google Scholar] [CrossRef]
- Tang, B.; Zhang, H.; Row, K.H. Application of deep eutectic solvents in the extraction and separation of target compounds from various samples. J. Sep. Sci. 2015, 38, 1053–1064. [Google Scholar] [CrossRef] [PubMed]
- Carriazo, D.; Serrano, M.C.; Gutiérrez, M.C.; Ferrer, M.L.; del Monte, F. Deep-eutectic solvents playing multiple roles in the synthesis of polymers and related materials. Chem. Soc. Rev. 2012, 41, 4996–5014. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahiri-Haghayegh, M.; Azizi, N. DESs as Catalyst. In Deep Eutectic Solvents: Synthesis, Properties, and Applications, 1st ed.; Ramón, D.J., Guillena, G., Eds.; Wiley-VCH: Weinheim, Germany, 2019; pp. 135–170. [Google Scholar]
- García-Álvarez, J.; Hevia, E.; Capriati, V. Reactivity of Polar Organometallic Compounds in Unconventional Reaction Media: Challenges and Opportunities. Eur. J. Org. Chem. 2015, 6779–6799. [Google Scholar] [CrossRef] [Green Version]
- Cicco, L.; Rodríguez-Álvarez, M.J.; Perna, F.M.; García-Álvarez, J.; Capriati, V. One-pot sustainable synthesis of tertiary alcohols by combining ruthenium-catalysed isomerisation of allylic alcohols and chemoselective addition of polar organometallic reagents in deep eutectic solvents. Green Chem. 2017, 19, 3069–3077. [Google Scholar] [CrossRef]
- García-Álvarez, J.; Hevia, E.; Capriati, V. The Future of Polar Organometallic Chemistry Written in Bio-Based Solvents and Water. Chem. Eur. J. 2018, 24, 14854–14863. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Li, X.T.; Ma, E.Q.; Mo, L.P.; Zhang, Z.H. Superparamagnetic CuFeO2 Nanoparticles in Deep Eutectic Solvent: An Efficient and Recyclable Catalytic System for the Synthesis of Imidazo[1,2-a]pyridines. ChemCatChem 2014, 6, 2854–2859. [Google Scholar] [CrossRef]
- Marset, X.; Khoshnood, A.; Sotorríos, L.; Gjmez-Bengoa, E.; Alonso, D.A.; Ramón, D.J. Deep Eutectic Solvent Compatible Metallic Catalysts: Cationic Pyridiniophosphine Ligands in Palladium Catalyzed Cross-Coupling Reactions. ChemCatChem 2017, 9, 1269–1275. [Google Scholar] [CrossRef] [Green Version]
- Ghinato, S.; Dilauro, G.; Perna, F.M.; Capriati, V.; Blangetti, M.; Prandi, C. Directed ortho-metalation–nucleophilic acyl substitution strategies in deep eutectic solvents: The organolithium base dictates the chemoselectivity. Chem. Commun. 2019, 55, 7741–7744. [Google Scholar] [CrossRef]
- Dilauro, G.; García, S.M.; Tagarelli, D.; Vitale, P.; Perna, F.M.; Capriati, V. Ligand-Free Bioinspired Suzuki–Miyaura Coupling Reactions using Aryltrifluoroborates as Effective Partners in Deep Eutectic Solvents. ChemSusChem 2018, 11, 3495–3501. [Google Scholar] [CrossRef]
- Quivelli, A.F.; Vitale, P.; Perna, F.M.; Capriati, V. Reshaping Ullmann Amine Synthesis in Deep Eutectic Solvents: A Mild Approach for Cu-Catalyzed C–N Coupling Reactions With No Additional Ligands. Front. Chem. 2019, 7, 723. [Google Scholar] [CrossRef] [PubMed]
- Messa, F.; Perrone, S.; Capua, M.; Tolomeo, F.; Troisi, L.; Capriati, V.; Salomone, A. Towards a sustainable synthesis of amides: Chemoselective palladium-catalysed aminocarbonylation of aryl iodides in deep eutectic solvents. Chem. Commun. 2018, 54, 8100–8103. [Google Scholar] [CrossRef] [PubMed]
- Messa, F.; Dilauro, G.; Perna, F.M.; Vitale, P.; Capriati, V.; Salomone, A. Sustainable Ligand-Free Heterogeneous Palladium-Catalyzed Sonogashira Cross-Coupling Reaction in Deep Eutectic Solvents. ChemCatChem 2020, (in press). [Google Scholar] [CrossRef]
- Marset, X.; Saavedra, B.; González-Gallardo, N.; Beaton, A.; León, M.M.; Luna, R.; Ramón, D.J.; Guillena, G. Palladium Mesoionic Carbene Pre-catalyst for general Cross-Coupling Transformations in Deep Eutectic Solvents. Front. Chem. 2019, 7, 700. [Google Scholar] [CrossRef] [PubMed]
- Meller, C.R.; Meiners, I.; Domínguez de María, P. Highly enantioselective tandem enzyme–organocatalyst crossed aldol reactions with acetaldehyde in deep-eutectic-solvents. RSC Adv. 2014, 4, 46097–46101. [Google Scholar] [CrossRef]
- Sheldon, A.R. Biocatalysis and Biomass Conversion in Alternative Reaction Media. Chem. Eur. J. 2016, 22, 12984–12999. [Google Scholar] [CrossRef]
- Vitale, P.; Abbinante, V.M.; Perna, F.M.; Salomone, A.; Cardellicchio, C.; Capriati, V. Unveiling the Hidden Performance of Whole Cells in the Asymmetric Bioreduction of Aryl-containing Ketones in Aqueous Deep Eutectic Solvents. Adv. Synth. Catal. 2017, 359, 1049–1057. [Google Scholar] [CrossRef]
- Vitale, P.; Perna, F.M.; Agrimi, G.; Pisano, I.; Mirizzi, F.; Capobianco, V.R.; Capriati, V. Whole-Cell Biocatalyst for Chemoenzymatic Total Synthesis of Rivastigmine. Catalysts 2018, 8, 55. [Google Scholar] [CrossRef] [Green Version]
- Cicco, L.; Ríos-Lombardía, N.; Rodríguez-Álvarez, M.J.; Moris, F.; Perna, F.M.; Capriati, V.; García-Álvarez, J.; González-Sabín, J. Programming cascade reactions interfacing biocatalysis with transition-metal catalysis in Deep Eutectic Solvents as biorenewable reaction media. Green Chem. 2018, 20, 3468–3475. [Google Scholar] [CrossRef]
- Martínez, R.; Berbegal, L.; Guillena, G.; Ramón, D.J. Bio-renewable enantioselective aldol reaction in natural deep eutectic solvents. Green Chem. 2016, 18, 1724–1730. [Google Scholar] [CrossRef] [Green Version]
- Massolo, E.; Palmieri, S.; Benaglia, M.; Capriati, V.; Perna, F.M. Stereoselective organocatalysed reactions in deep eutectic solvents: Highly tunable and biorenewable reaction media for sustainable organic synthesis. Green Chem. 2016, 18, 792–797. [Google Scholar] [CrossRef]
- Brenna, D.; Massolo, E.; Puglisi, A.; Rossi, S.; Celentano, G.; Benaglia, M.; Capriati, V. Towards the development of continuous, organocatalytic, and stereoselective reactions in deep eutectic solvents. Beilstein J. Org. Chem. 2016, 12, 2620–2626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torregrosa-Chinillach, A.; Sánchez-Laó, A.; Santagostino, E.; Chinchilla, R. Organocatalytic Asymmetric Conjugate Addition of Aldehydes to Maleimides and Nitroalkenes in Deep Eutectic Solvents. Molecules 2019, 24, 4058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milla, L.; Dall’Asta, V.; Ferrara, C.; Berbenni, V.; Quartarone, E.; Perna, F.M.; Capriati, V.; Mustarelli, P. Bio-inspired choline chloride-based deep eutectic solvents as electrolytes for lithium-ion batteries. Solid State Ionics 2018, 323, 44–48. [Google Scholar] [CrossRef]
- Milano, F.; Giotta, L.; Guascito, M.R.; Agostiano, A.; Sblendorio, S.; Valli, L.; Perna, F.M.; Cicco, L.; Trotta, M.; Capriati, V. Functional Enzymes in Nonaqueous Environment: The Case of Photosynthetic Reaction Centers in Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2017, 5, 7768–7776. [Google Scholar] [CrossRef]
- Boldrini, C.L.; Manfredi, N.; Perna, F.M.; Trifiletti, V.; Capriati, V.; Abbotto, A. Dye-Sensitized Solar Cells that use an Aqueous Choline Chloride-Based Deep Eutectic Solvent as Effective Electrolyte Solution. Energy Technol. 2017, 5, 345–353. [Google Scholar] [CrossRef]
- Boldrini, C.L.; Manfredi, N.; Perna, F.M.; Capriati, V.; Abbotto, A. Designing Eco-Sustainable Dye-Sensitized Solar Cells by the Use of a Menthol-Based Hydrophobic Eutectic Solvent as an Effective Electrolyte Medium. Chem. Eur. J. 2018, 24, 17656–17659. [Google Scholar] [CrossRef]
- Capua, M.; Perrone, S.; Perna, F.M.; Vitale, P.; Troisi, L.; Salomone, A.; Capriati, V. An Expeditious and Greener Synthesis of 2-Aminoimidazoles in Deep Eutectic Solvents. Molecules 2016, 21, 924. [Google Scholar] [CrossRef] [Green Version]
- Mancuso, R.; Maner, A.; Cicco, L.; Perna, F.M.; Capriati, V.; Gabriele, B. Synthesis of thiophenes in a deep eutectic solvent: Heterocyclodehydration and iodocyclization of 1-mercapto-3-yn-2-ols in a choline chloride/glycerol medium. Tetrahedron 2016, 72, 4239–4244. [Google Scholar] [CrossRef]
- Dilauro, G.; Quivelli, A.F.; Vitale, P.; Capriati, V.; Perna, F.M. Water and sodium chloride: Essential ingredients for robust and fast Pd-catalysed cross-coupling reactions between organolithium reagents and (hetero)aryl halides. Angew. Chem. Int. Ed. 2019, 58, 1799–1802. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Mook-Jung, I.; Koh, J.-Y. Histochemically Reactive Zinc in Plaques of the Swedish Mutant β-Amyloid Precursor Protein Transgenic Mice. J. Neurosci. 1999, 19, RC10. [Google Scholar] [CrossRef] [PubMed]
- Lovell, M.A.; Robertson, J.D.; Teesdale, W.J.; Campbell, J.L.; Markesbery, W.R. Copper, iron and zinc in Alzheimer’s disease senile plaques. J. Neurol. Sci. 1998, 158, 47–52. [Google Scholar] [CrossRef]
- Suh, S.W.; Jensen, K.B.; Jensen, M.S.; Silva, D.S.; Kesslak, P.J.; Danscher, G.; Frederickson, C.J. Histochemically-reactive zinc in amyloid plaques, angiopathy, and degenerating neurons of Alzheimer’s diseased brains. Brain Res. 2000, 852, 274–278. [Google Scholar] [CrossRef]
- Liang, S.H.; Southon, A.G.; Fraser, B.H.; Krause-Heuer, A.M.; Zhang, B.; Shoup, T.M.; Lewis, R.; Volitakis, I.; Han, Y.; Greguric, I.; et al. Novel Fluorinated 8-Hydroxyquinoline Based Metal Ionophores for Exploring the Metal Hypothesis of Alzheimer’s Disease. ACS Med. Chem. Lett. 2015, 6, 1025–1029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cherny, R.A.; Atwood, C.S.; Xilinas, M.E.; Gray, D.N.; Jones, W.D.; McLean, C.A.; Barnham, K.J.; Volitakis, I.; Fraser, F.W.; Kim, Y.-S.; et al. Treatment with a Copper-Zinc Chelator Markedly and Rapidly Inhibits Aβ-Amyloid Accumulation in Alzheimer’s Disease Transgenic Mice. Neuron 2001, 30, 665–676. [Google Scholar] [CrossRef] [Green Version]
- Zagidullin, R.N. Reactions of N-(β-aminoethyl)piperazine and its derivatives. Chem. Heterocycl. Comp. 1991, 27, 309–312. [Google Scholar] [CrossRef]
- Yusof, R.; Abdulmalek, E.; Sirat, K.; Basyaruddin, M.; Rahman, A. Tetrabutylammonium Bromide (TBABr)-Based Deep Eutectic Solvents (DESs) and Their Physical Properties. Molecules 2014, 19, 8011–8026. [Google Scholar] [CrossRef]
- Mjalli, F.S.; Naser, J.; Jibril, B.; Alizadeh, V.; Gano, Z. Tetrabutylammonium Chloride Based Ionic Liquid Analogues and Their Physical Properties. J. Chem. Eng. Data 2014, 59, 2242–2251. [Google Scholar] [CrossRef]
- Warrag, S.E.E.; Kroon, M.C. Hydrophobic Deep Eutectic Solvents. In Deep Eutectic Solvents: Synthesis, Properties, and Applications, 1st ed.; Ramón, D.J., Guillena, G., Eds.; Wiley-VCH: Weinheim, Germany, 2019; pp. 83–93. [Google Scholar]
- Porcelli, L.; Gilardi, F.; Laghezza, A.; Piemontese, L.; Mitro, N.; Azzariti, A.; Altieri, F.; Cervoni, L.; Fracchiolla, G.; Giudici, M.; et al. Synthesis, characterization and biological evaluation of ureidofibrate-like derivatives endowed with peroxisome proliferator-activated receptor activity. J. Med. Chem. 2012, 55, 37–54. [Google Scholar] [CrossRef]
- Available online: https://www.alzforum.org/therapeutics/clioquinol (accessed on 11 November 2019).
- Baldisserotto, A.; Demurtas, M.; Lampronti, I.; Tacchini, M.; Moi, D.; Balboni, G.; Pacifico, S.; Vertuani, S.; Manfredini, S.; Onnis, V. Synthesis and evaluation of antioxidant and antiproliferative activity of 2-arylbenzimidazoles. Bioorg. Chem. 2019, (in press). [Google Scholar] [CrossRef]
- Karthikeyan, C.; Solomon, V.R.; Lee, H.; Trivedi, P. Synthesis and biological evaluation of 2-(phenyl)-3H-benzo[d]imidazole-5-carboxylic acids and its methyl esters as potent anti-breast cancer agents. Arab. J. Chem. 2017, 10, S1788–S1794. [Google Scholar] [CrossRef] [Green Version]
- Di Gioia, M.L.; Cassano, R.; Costanzo, P.; Herrera Cano, N.; Maiuolo, L.; Nardi, M.; Nicoletta, F.P.; Oliverio, M.; Procopio, A. Green Synthesis of Privileged Benzimidazole Scaffolds Using Active Deep Eutectic Solvent. Settings. Molecules 2019, 24, 2885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Sample Availability: Samples of the compounds are not available from the authors. |



| Entry | Base-1 (Equiv) | Base-2 (Equiv) | BnBr (Equiv) | T (°C) | T (h) | Solvent | 3, Yield (%) b |
|---|---|---|---|---|---|---|---|
| 1 | TEA (1.4) | K2CO3 (6.6) | 2.3 | 50 | 3 | CH3CN | 64 c |
| 2 | KOH (1.1) | - | 1.0 | 50 | 24 | ChCl/gly | NR d |
| 3 | KOH (1.1) | - | 1.0 | 50 | 24 | ChCl/urea | NR d |
| 4 | t-BuOK (1.1) | - | 1.0 | 50 | 24 | ChCl/gly | 24 |
| 5 | t-BuOK (1.1) | - | 1.0 | 50 | 24 | ChCl/urea | 10 |
| 6 | t-BuOK (1.1) | - | 1.0 | 50 | 24 | ChCl/PG | 20 |
| 7 | t-BuOK (1.1) | - | 1.0 | 100 | 24 | d-fructose/ChCl | 8 |
| 8 | t-BuOK (1.1) | - | 1.0 | 100 | 24 | d-fructose /urea | 8 |
| 9 | t-BuOK (1.1) | - | 1.0 | 50 | 24 | ChCl/gly | 24 |
| 10 | t-BuOK (1.1) | - | 1.0 | 50 | 24 | ChCl/urea | 10 |
| 11 | TEA (1.4) | K2CO3 (6.6) | 2.3 | 50 | 24 | ChCl/gly | NR d |
| 12 | K2CO3 (2.0) | - | 1.0 | 50 | 24 | ChCl/gly | NR d |
| 13 | TEA (2.0) | - | 1.0 | 50 | 24 | ChCl/gly | 26 |
| 14 | TEA (2.0) | - | 1.0 | 50 | 24 | ChCl/PG | 44 |
| 15 | TEA (2.0) | - | 2.0 | 50 | 24 | ChCl/PG | 64 c |
| 16 | TEA (2.0) | - | 2.0 | 50 | 24 | ChCl/gly | 61 c |
| 17 | TEA (2.0) | - | 1.0 | 50 | 24 | DL-menthol/LA | NR d |
| 18 | TEA (2.0) | - | 2.0 | 50 | 24 | Bu4NBr/gly | 44 c |
| 19 | TEA (2.0) | - | 2.0 | 50 | 24 | Bu4NCl/gly | 68 c |

| Entry | T (°C) | T (h) | Oxidant | Solvent | 7a, Yield (%) b |
|---|---|---|---|---|---|
| 1 | 100 | 0.5 | Na2S2O5 | ChCl/urea | 66 |
| 2 | 100 | 0.5 | Na2S2O5 | ChCl/gly | 80 |
| 3 | 100 | 0.5 | Na2S2O5 | ChCl/LA | 76 |
| 4 | 50 | 0.5 | Na2S2O5 | ChCl/gly | 84 |
| 5 | 25 | 24 | Na2S2O5 | ChCl/gly | 36 |
| 6 | 50 | 0.5 | urea-H2O2 | ChCl/gly | 17 c |
| 7 | 50 | 0.5 | - d | ChCl/gly | 29 c |
| 8 | 100 | 12 | Na2S2O5 | DMA | 67 |

| Entry | Reagent 1 (Equiv) | Reagent 2 (Equiv) | T (°C) | Solvent | 8, Yield (%) b |
|---|---|---|---|---|---|
| 1 | NHS (1) | DCC (1) | 25 | DMF | 21 |
| 2 | NHS (1) | DCC (1) | 60 | ChCl/gly | 16 |
| 3 | NHS (1) | DCC (1) | 60 | ChCl/PG | 30 |
| 4 | NHS (1) | DCC (1) | 60 | ChCl/urea | 13 |
| 5 | NHS (1) | DCC (1) | 60 | menthol/LA | NR c |
| 6 | NHS (1) | DCC (1) | 60 | d-fructose/urea | NR c |
| 7 | NHS (1) | DCC (1) | 60 | Bu4NBr/gly | 7 d |
| 8 | NHS (1) | DCC (1) | 60 | Bu4NCl/gly | 19 d |
| 9 | NHS (1) | - | 60 | ChCl/PG | <5 d |
| 10 | - | DCC (1) | 60 | ChCl/PG | NR c |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piemontese, L.; Sergio, R.; Rinaldo, F.; Brunetti, L.; Perna, F.M.; Santos, M.A.; Capriati, V. Deep Eutectic Solvents as Effective Reaction Media for the Synthesis of 2-Hydroxyphenylbenzimidazole-Based Scaffolds en Route to Donepezil-Like Compounds. Molecules 2020, 25, 574. https://doi.org/10.3390/molecules25030574
Piemontese L, Sergio R, Rinaldo F, Brunetti L, Perna FM, Santos MA, Capriati V. Deep Eutectic Solvents as Effective Reaction Media for the Synthesis of 2-Hydroxyphenylbenzimidazole-Based Scaffolds en Route to Donepezil-Like Compounds. Molecules. 2020; 25(3):574. https://doi.org/10.3390/molecules25030574
Chicago/Turabian StylePiemontese, Luca, Roberta Sergio, Federica Rinaldo, Leonardo Brunetti, Filippo M. Perna, M. Amélia Santos, and Vito Capriati. 2020. "Deep Eutectic Solvents as Effective Reaction Media for the Synthesis of 2-Hydroxyphenylbenzimidazole-Based Scaffolds en Route to Donepezil-Like Compounds" Molecules 25, no. 3: 574. https://doi.org/10.3390/molecules25030574
APA StylePiemontese, L., Sergio, R., Rinaldo, F., Brunetti, L., Perna, F. M., Santos, M. A., & Capriati, V. (2020). Deep Eutectic Solvents as Effective Reaction Media for the Synthesis of 2-Hydroxyphenylbenzimidazole-Based Scaffolds en Route to Donepezil-Like Compounds. Molecules, 25(3), 574. https://doi.org/10.3390/molecules25030574