Laser-Plasma Spatiotemporal Cyanide Spectroscopy and Applications
Abstract
:1. Introduction
2. Experiment Details
3. Results and Discussion
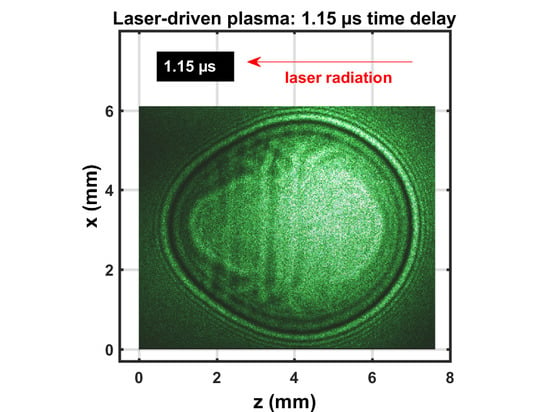
3.1. Shadowgraphs
3.2. Cyanide Spectra
3.3. Cyanide Temperature
3.4. Electron Density
3.5. Cyanide Spectra in Flowing Gas
3.6. Abel-Inverted Spectra
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Parigger, C.G.; Helstern, C.M.; Gautam, G. Molecular Emission Spectroscopy of Cyanide in Laser-induced Plasma. Int. Rev. At. Mol. Phys. 2017, 8, 25–35. [Google Scholar]
- Fent, K.W.; Evans, D.E.; Babik, K.; Striley, C.; Bertke, S.; Kerber, S.; Smith, D.; Horn, G.P. Airborne contaminants during controlled residential fires. J. Occup. Environ. Hyg. 2018, 15, 399–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arslanov, D.D.; Castro, M.P.P.; Creemers, N.A.; Neerincx, A.H.; Spunei, M.; Mandon, J.; Cristescu, S.M.; Merkus, P.; Harren, F.J.M. Optical parametric oscillator-based photoacoustic detection of hydrogen cyanide for biomedical applications. J. Biomed. Opt. 2013, 18, 107002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ambrose, J.L.; Zhou, Y.; Haase, K.; Mayne, H.R.; Talbot, R.; Sive, B.C. A gas chromatographic instrument for measurement of hydrogen cyanide in the lower atmosphere. Atmos. Meas. Tech. 2012, 5, 1229–1240. [Google Scholar] [CrossRef] [Green Version]
- Mudder, T.I.; Botz, M.M. Cyanide and society: A critical review. Eur. J. Miner. Process. Environ. Protect. 2004, 4, 62–74. [Google Scholar]
- Bolstad-Johnson, D.M.; Burgess, J.L.; Crutchfield, C.D.; Storment, S.; Gerkin, R.; Wilson, J.R. Characterization of firefighter exposures during fire overhaul. Am. Ind. Hyg. Assoc. 2000, 61, 636–641. [Google Scholar] [CrossRef]
- Betke, T.; Rommelmann, P.; Oike, K.; Asano, Y.; Groger, H. Cyanide-Free and Broadly Applicable Enantioselective Synthetic Platform for Chiral Nitriles through a Biocatalytic Approach. Angew. Chem. Int. Edit. 2017, 56, 12361–12366. [Google Scholar] [CrossRef]
- Metzner, R.; Okazaki, S.; Asano, Y.; Groger, H. Cyanide-free Enantioselective Synthesis of Nitriles: Synthetic Proof of a Biocatalytic Concept and Mechanistic Insights. Chem. Cat. Chem 2014, 6, 3105–3109. [Google Scholar] [CrossRef]
- Plass, C.; Hinzmann, A.; Terhorst, M.; Brauer, W.; Oike, K.; Yavuzer, H.; Asano, Y.; Vorholt, A.; Betke, T.; Gröger, H. Approaching Bulk Chemical Nitriles from Alkenes: A Hydrogen Cyanide-Free Approach through a Combination of Hydroformylation and Biocatalysis. Am. Chem. Soc. Catal. 2019, 9, 5198–5203. [Google Scholar] [CrossRef]
- Hilson, G.; Monhemius, A.J. Alternatives to cyanide in the gold mining industry: What prospects for the future. J. Clean. Prod. 2006, 14, 1158–1167. [Google Scholar] [CrossRef]
- Karlsson, H.L. Ammonia, nitrous oxide and hydrogen cyanide emissions from five passenger vehicles. Sci. Total Environ. 2004, 334−335, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Mekuto, L.; Ntwampe, S.K.O.; Akcil, A. An integrated biological approach for treatment of cyanidation wastewater. Sci. Total Environ. 2014, 571, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Akcil, A.; Erust, C.; Gahan, C.S.; Ozgun, M.; Sahin, M.; Tuncuk, A. Precious metal recovery from waste printed circuit boards using cyanide and non-cyanide lixiviants—A review. Waste Manag. 2015, 45, 258–271. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, R.; Ordóñez, A.; Loredo, J.; Younger, P.L. Wetland-based passive treatment systems for gold ore processing effluents containing residual cyanide, metals and nitrogen species. Environ. Sci. Process. Impacts 2013, 15, 2115–2124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moussa, S.G.; Leithead, A.; Li, S.-M.; Chan, T.W.; Wentzell, J.J.B.; Stroud, C.; Zhang, J.; Lee, P.; Jeffery, G.L.; Brook, R.; et al. Emissions of hydrogen cyanide from on-road gasoline and diesel vehicles. Atmos. Environ. 2016, 131, 185–195. [Google Scholar] [CrossRef] [Green Version]
- Baum, M.M.; Moss, J.A.; Pastel, S.H.; Poskrebyshev, G.A. Hydrogen cyanide exhaust emissions from in-use motor vehicles. Environ. Sci. Technol. 2007, 41, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Merten, J.; Jones, M.; Hoke, S.; Allen, S. Differential Spectral Imaging of the CN Violet Band in Laser-Induced Plasmas on TNT Simulant Molecules. Phys. Conf. Ser. 2014, 548, 012042. [Google Scholar] [CrossRef] [Green Version]
- Dagaut, P.; Glarborg, P.; Alzueta, M.U. The oxidation of hydrogen cyanide and related chemistry. Prog. Energy Combust. 2008, 34, 1–46. [Google Scholar] [CrossRef]
- Cremers, D.A.; Radziemski, L.J. Handbook of Laser-Induced Breakdown Spectroscopy; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2006. [Google Scholar]
- Gautam, G.; Helstern, C.M.; Drake, K.A.; Parigger, C.G. Imaging of Laser-induced Plasma Expansion Dynamics in Ambient Air. Int. Rev. At. Mol. Phys. 2016, 7, 45–51. [Google Scholar]
- Taylor, G.I. The formation of a blast wave by a very intense explosion.—II. The atomic explosion of 1945. Proc. Roy. Soc. A 1950, 201, 175–186. [Google Scholar]
- Thiyagarajan, M.; Thompson, S. Optical breakdown threshold investigation of 1064 nm laser induced air plasmas. J. Appl. Phys. 2012, 111, 073302. [Google Scholar] [CrossRef]
- Kogelnik, H.; Li, T. Laser Beams and Resonators. Appl. Opt. 1966, 5, 1550–1567. [Google Scholar] [CrossRef] [PubMed]
- Technical Note: Gaussian Beam Optics. Available online: https://www.newport.com/n/gaussian-beam-optics (accessed on 11 January 2020).
- Nelder, J.A.; Mead, R. A simplex method for function minimization. Comput. J. 1965, 7, 308–313. [Google Scholar] [CrossRef]
- Parigger, C.G.; Woods, A.C.; Surmick, D.M.; Gautam, G.; Witte, M.J.; Hornkohl, J.O. Computation of diatomic molecular spectra for selected transitions of aluminum monoxide, cyanide, diatomic carbon, and titanium monoxide. Spectrochim. Acta Part B At. Spectrosc. 2015, 107, 132–138. [Google Scholar] [CrossRef]
- Parigger, C.G.; Hornkohl, J.O. Quantum Mechanics of the Diatomic Molecule with Applications; IOP Publishing: Bristol, UK, 2019. [Google Scholar]
- Dackman, M. Laser-Induced Breakdown Spectroscopy for Analysis of High-Density Methane-Oxygen Mixtures. Master’s Thesis, University of Tennessee, Knoxville, TN, USA, 2014. [Google Scholar]
- Griem, H.R. Spectral Line Broadening by Plasmas; Academic Press: New York, NY, USA, 1974. [Google Scholar]
- Parigger, C.G.; Gautam, G.; Surmick, D.M. Radial electron density measurements in laser-induced plasma from Abel inverted hydrogen Balmer beta line profiles. Int. Rev. At. Mol. Phys. 2015, 6, 43–55. [Google Scholar]
- Parigger, C.G.; Surmick, D.M.; Gautam, G. Self-absorption characteristics of measured laser-induced plasma line shapes. Phys. Conf. Ser. 2017, 810, 012012. [Google Scholar] [CrossRef]
- Pretzler, G. A New Method for Numerical Abel-Inversion. Z. Naturforsch. 1991, 46a, 639–641. [Google Scholar] [CrossRef] [Green Version]
- Pretzler, G.; Jäger, H.; Neger, T.; Philipp, H.; Woisetschläger, J. Comparison of Different Methods of Abel Inversion Using Computer Simulated and Experimental Side-On Data. Z. Naturforsch. 1992, 47a, 955–970. [Google Scholar]
Sample Availability: Not available. |













| Time Delay (ns) | r (mm) for Air ( kg/m) | R (mm) for CN Mix ( kg/m) |
|---|---|---|
| 200 | 1.40 | 1.31 |
| 450 | 1.93 | 1.82 |
| 700 | 2.31 | 2.17 |
| 950 | 2.61 | 2.45 |
| 1200 | 2.86 | 2.69 |
| 1450 | 3.09 | 2.90 |
| Time Delay (ns) | r (mm) for Air ( kg/m) | R (mm) for CN Mix ( kg/m) |
|---|---|---|
| 200 | 1.46 | 1.37 |
| 450 | 2.02 | 1.90 |
| 700 | 2.41 | 2.27 |
| 950 | 2.73 | 2.56 |
| 1200 | 2.99 | 2.81 |
| 1450 | 3.23 | 3.04 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parigger, C.G.; Helstern, C.M.; Jordan, B.S.; Surmick, D.M.; Splinter, R. Laser-Plasma Spatiotemporal Cyanide Spectroscopy and Applications. Molecules 2020, 25, 615. https://doi.org/10.3390/molecules25030615
Parigger CG, Helstern CM, Jordan BS, Surmick DM, Splinter R. Laser-Plasma Spatiotemporal Cyanide Spectroscopy and Applications. Molecules. 2020; 25(3):615. https://doi.org/10.3390/molecules25030615
Chicago/Turabian StyleParigger, Christian G., Christopher M. Helstern, Benjamin S. Jordan, David M. Surmick, and Robert Splinter. 2020. "Laser-Plasma Spatiotemporal Cyanide Spectroscopy and Applications" Molecules 25, no. 3: 615. https://doi.org/10.3390/molecules25030615
APA StyleParigger, C. G., Helstern, C. M., Jordan, B. S., Surmick, D. M., & Splinter, R. (2020). Laser-Plasma Spatiotemporal Cyanide Spectroscopy and Applications. Molecules, 25(3), 615. https://doi.org/10.3390/molecules25030615