Smart, Tunable CQDs with Antioxidant Properties for Biomedical Applications—Ecofriendly Synthesis and Characterization
Abstract
:1. Introduction
2. Results
2.1. FourierTransform Infrared Spectroscopy (FTIR) and Nuclear Magnetic Resonanse (NMR) Analysis of the Modified Carbon Quantum Dots
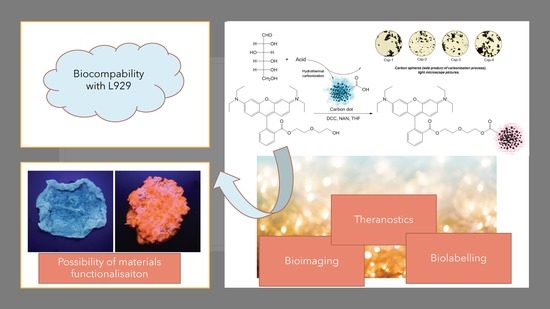
2.2. Morphology Study
2.3. Spectroscopic Properties Study
2.4. Antioxidant Activity Study
2.5. Cytotoxicity Study
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. CQDs Preparation and Modification
3.2.2. Optical Properties Study
3.2.3. Antioxidant Study
3.2.4. Cytotoxicity Study
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xu, L.; Zhang, Y.; Pan, H.; Xu, N.; Mei, C.; Mao, H.; Zhang, W.; Cai, J.; Xu, C. Preparation and Performance of Radiata-Pine-Derived Polyvinyl Alcohol/Carbon Quantum Dots Fluorescent Films. Materials 2020, 13, 67. [Google Scholar] [CrossRef] [Green Version]
- Molaei, M.J. A review on nanostructured carbon quantum dots and their applications in biotechnology, sensors, and chemiluminescence. Talanta 2019, 196, 456–478. [Google Scholar] [CrossRef]
- Deerinck, T.J. The application of fluorescent quantum dots to confocal, multiphoton, and electron microscopic imaging. Toxicol. Pathol. 2008, 36, 112–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Feng, Y.; Dong, P.; Huang, J. A Mini Review on Carbon Quantum Dots: Preparation, Properties, and Electrocatalytic Application. Front. Chem. 2019, 7, 671. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Zhang, M.; Bhandari, B.; Yang, C. Food waste as a carbon source in carbon quantum dots technology and their applications in food safety detection. Trends Food Sci. Technol. 2020, 95, 86–96. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, X.; Wang, M.; Huang, J.; Jiang, X.; Pang, J.; Xu, F.; Zhang, X. Synthesis of N-doped carbon quantum dots from bio-waste lignin for selective irons detection and cellular imaging. Int. J. Biol. Macromol. 2019, 128, 537–545. [Google Scholar] [CrossRef]
- Anas, N.A.A.; Fen, Y.W.; Omar, N.A.S.; Daniyal, W.M.E.M.M.; Ramdzan, N.S.M.; Saleviter, S. Development of Graphene Quantum Dots-Based Optical Sensor for Toxic Metal Ion Detection. Sensors 2019, 19, 3850. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Liu, Y.; Yin, Y.; Li, H.; Huang, J. Facile ultrasonic synthesized NH2-carbon quantum dots for ultrasensitive Co2+ ion detection and cell imaging. Talanta 2019, 205, 120121. [Google Scholar] [CrossRef]
- Boakye-Yiadom, K.O.; Kesse, S.; Opoku-Damoah, Y.; Filli, M.S.; Aquib, M.; Joelle, M.B.; Farooq, M.A.; Mavlyanova, R.; Raza, F.; Bavi, R.; et al. Carbon dots: Applications in bioimaging and theranostics. Int. J. Pharm. 2019, 564, 308–317. [Google Scholar] [CrossRef]
- Wu, F.; Su, H.; Wang, K.; Wong, W.-K.; Zhu, X. Facile synthesis of N-rich carbon quantum dots from porphyrins as efficient probes for bioimaging and biosensing in living cells. Int. J. Nanomedicine. 2017, 12, 7375–7391. [Google Scholar] [CrossRef] [Green Version]
- Ayoubi, M.; Naserzadeh, P.; Hashemi, M.T.; Rostami, M.R.; Tamjid, E.; Tavakoli, M.M.; Simchi, A. Biochemical mechanisms of dose-dependent cytotoxicity and ROS-mediated apoptosis induced by lead sulfide/graphene oxide quantum dots for potential bioimaging applications. Sci. Rep. 2017, 7, 12896. [Google Scholar] [CrossRef]
- Chinnathambi, S.; Shirahata, N. Recent advances on fluorescent biomarkers of near-infrared quantum dots for in vitro and in vivo imaging. Sci. Technol. Adv. Mater. 2019, 20, 337–355. [Google Scholar] [CrossRef] [Green Version]
- Hong, G.-L.; Zhao, H.-L.; Deng, H.-H.; Yang, H.-J.; Peng, H.-P.; Liu, Y.-H.; Chen, W. Fabrication of ultra-small monolayer graphene quantum dots by pyrolysis of trisodium citrate for fluorescent cell imaging. Int. J. Nanomedicine. 2018, 13, 4807–4815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matea, C.T.; Mocan, T.; Tabaran, F.; Pop, T.; Mosteanu, O.; Puia, C.; Iancu, C.; Mocan, L. Quantum dots in imaging, drug delivery and sensor applications. Int. J. Nanomed. 2017, 12, 5421–5431. [Google Scholar] [CrossRef] [Green Version]
- Chu, K.-W.; Lee, S.L.; Chang, C.-J.; Liu, L. Recent Progress of Carbon Dot Precursors and Photocatalysis Applications. Polymers 2019, 11, 689. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Hu, C.; Yang, Y.; Cui, J.; Liu, Y. Rapid Synthesis of Carbon Dots by Hydrothermal Treatment of Lignin. Materials 2016, 9, 184. [Google Scholar] [CrossRef]
- Abazar, F.; Noorbakhsh, A. Chitosan-carbon quantum dots as a new platform for highly sensitive insulin impedimetric aptasensor. Sens. Actuator B-Chem. 2020, 304, 127281. [Google Scholar] [CrossRef]
- Zhang, W.; Wu, B.; Li, Z.; Wang, Y.; Zhou, J.; Li, Y. Carbon quantum dots as fluorescence sensors for label-free detection of folic acid in biological samples. Spectrochim. Spectrochimica. Acta Part A 2020, 229, 117931. [Google Scholar] [CrossRef]
- Garg, B.; Bisht, T. Carbon Nanodots as Peroxidase Nanozymes for Biosensing. Molecules 2016, 21, 1653. [Google Scholar] [CrossRef]
- Pan, J.; Zheng, Z.; Yang, J.; Wu, Y.; Lu, F.; Chen, Y.; Gao, W. A novel and sensitive fluorescence sensor for glutathione detection by controlling the surface passivation degree of carbon quantum dots. Talanta 2017, 166, 1–7. [Google Scholar] [CrossRef]
- Alarfaj, N.A.; El-Tohamy, M.F.; Oraby, H.F. CA 19-9 Pancreatic Tumor Marker Fluorescence Immunosensing Detection via Immobilized Carbon Quantum Dots Conjugated Gold Nanocomposite. Int. J. Mol. Sci. 2018, 19, 1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.F.; Yan, Z.-K.; Chen, L.-B.; Jin, J.-P.; Li, D.-D. Desmin detection by facile prepared carbon quantum dots for early screening of colorectal cancer. Medicine 2017, 96, e5521. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.-W.; Hsieh, M.-L.; Liu, W.-R. A facile approach to synthesize carbon quantum dots with pH-dependent properties. Dyes Pigm. 2019, 169, 73–80. [Google Scholar] [CrossRef]
- Devia, P.; Sainia, S.; Kim, K.-H. The advanced role of carbon quantum dots in nanomedical applications. Biosens. Bioelectron. 2019, 141, 111158. [Google Scholar] [CrossRef]
- Reshma, V.G.; Mohanan, P.V. Quantum dots: Applications and safety consequences. J. Lumin. 2019, 205, 287–298. [Google Scholar] [CrossRef]
- Wagner, A.M.; Knipe, J.M.; Orive, G.; Peppas, N.A. Quantum dots in biomedical applications. Acta Biomater. 2019, 94, 44–63. [Google Scholar] [CrossRef]
- Vieira, S.; Vial, S.; Reis, R.L.; Oliveira, J.M. Nanoparticles for Bone Tissue Engineering. Biotechnol. Prog. 2017, 33, 590–611. [Google Scholar] [CrossRef] [Green Version]
- Ranjbar-Navazi, Z.; Omidi, Y.; Eskandani, M.; Davaran, S. Cadmium-free quantum dot-based theranostics. TrAC 2019, 118, 386–400. [Google Scholar] [CrossRef]
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J. Am Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef]
- Das, R.; Bandyopadhyay, R.; Pramanik, P. Carbon quantum dots from natural resource: A review. Mater. Today Chem. 2018, 8, 96–109. [Google Scholar] [CrossRef]
- Tejwan, N.; Saha, S.K.; Das, J. Multifaceted applications of green carbon dots synthesized from renewable sources. Adv. Colloid Interface Sci. 2020, 275, 102046. [Google Scholar] [CrossRef] [PubMed]
- Namdari, P.; Negahdari, B.; Eatemadi, A. Synthesis, properties and biomedical applications of carbon-based quantum dots: An updated review. Biomed. Pharmacother. 2017, 87, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Kumar, R.; Singh, D.P.; Savu, R.; Moshkalev, S.A. Progress in microwave-assisted synthesis of quantum dots (graphene/carbon/semiconducting) for bioapplications: A review. Mater. Today Chem. 2019, 12, 282–314. [Google Scholar] [CrossRef]
- Sun, X.; Liu, X.; Yang, L.; Wang, X.; Yang, W.; Wei, M.; Liu, X.; Cao, J.; Yang, J.; Xing, S.G. Tailoring Blue-Green Double Emissions in Carbon Quantum Dots via Co-Doping Engineering by Competition Mechanism between Chlorine-Related States and Conjugated π-Domains. Nanomaterials 2018, 8, 635. [Google Scholar] [CrossRef] [Green Version]
- Yadegari, A.; Khezri, J.; Esfandiari, S.; Mahdavi, H.; Karkhane, A.A.; Rahighid, R.; Heidarimoghadam, R.; Tayebi, L.; Hashemi, E.; Farmany, A. Bottom-up synthesis of nitrogen and oxygen co-decorated carbon quantum dots with enhanced DNA plasmid expression. Colloids Surf. B 2019, 184, 110543. [Google Scholar] [CrossRef]
- Madani, S.Y.; Shabani, F.; Dwek, M.V.; Seifalian, A.M. Conjugation of quantum dots on carbon nanotubes for medical diagnosis and treatment. Int. J. Nanomed. 2013, 8, 941–950. [Google Scholar]
- Kulkarni, N.S.; Guererro, Y.; Gupta, N.; Muth, A.; Gupta, V. Exploring potential of quantum dots as dual modality for cancer therapy and diagnosis. J. Drug Deliv. Sci. Technol. 2019, 49, 352–364. [Google Scholar] [CrossRef]
- Cailotto, S.; Mazzaro, R.; Enrichi, F.; Vomiero, A.; Selva, M.; Cattaruzza, E.; Cristofori, D.; Amadio, E.; Perosa, A. Design of Carbon Dots for Metal-free Photoredox Catalysis. ACS Appl. Mater. Interfaces 2018, 10, 40560–40567. [Google Scholar] [CrossRef]
- Hutton, G.A.; Martindale, B.C.; Reisner, E. Carbon dots as photosensitisers for solar-driven catalysis. Chem. Soc. Rev. 2017, 46, 6111–6123. [Google Scholar] [CrossRef] [Green Version]
- Umashankar, A.; Corenblum, M.J.; Ray, S.; Valdez, M.; Yoshimaru, E.S.; Trouard, T.P.; Madhavan, L. Effects of the iron oxide nanoparticle Molday ION Rhodamine B on the viability and regenerative function of neural stem cells: relevance to clinical translation. Int. J. Nanomed. 2016, 11, 1731–1748. [Google Scholar]
- Bartholomä, M.D.; He, H.; Pacak, C.; Dunning, P.; Fahey, F.H.; McGowan, F.; Cowan, D.; Treves, T.; Packard, A.B. Biological Characterization of F-18-Labeled Rhodamine B, a Potential Positron Emission Tomography Perfusion Tracer. Nucl. Med. Biol. 2013, 40, 1043–1048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, B.J.; Mitchell, S.N.; Paton, C.J.; Stevenson, J.; Staunton, K.M.; Snoad, N.; Beebe, N.; White, B.J.; Ritchie, S.A. Use of rhodamine B to mark the body and seminal fluid of male Aedes aegypti for mark-release-recapture experiments and estimating efficacy of sterile male releases. PLoS Negl. Trop. Dis. 2017, 11, e0005902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, K.; Qing, W.; Hu, W.; Lu, M.; Wang, Y.; Liu, X. On-off-on fluorescent carbon dots from waste tea: Their properties, antioxidant and selective detection of CrO42−, Fe3+, ascorbic acid and L-cysteine in real samples. Spectrochim. Spectrochimica. Acta Part A 2019, 213, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Janus, Ł.; Piątkowski, M.; Radwan-Pragłowska, J. Microwave-Assisted Synthesis and Characterization of Poly(L-lysine)-Based Polymer/Carbon Quantum Dot Nanomaterials for Biomedical Purposes. Materials 2019, 12, 3825. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of the compounds are available from the authors on the request. |











| 1H-NMR | Chemical Shift, ppm | 13C-NMR | Chemical Shift, ppm |
|---|---|---|---|
| a | 1.27 | a | 11.4 |
| b | 1.27 | b | 45.5 |
| c | 8.33 | c | 95.6 |
| d | 7.85 | d | 114.1 |
| e | 7.79 | e | 64.4 |
| f | 7.78 | f | 68.3 |
| g | 7.40 | g | 72.1 |
| h | 3.33 | h | 60.6 |
| i | 3.35 | i | 113.5 |
| j | 3.95 | j | 132.7 |
| k | 4.10 | k | 158.0 |
| l | 6.97 | l | 131.1 |
| m | 7.09 | m | 156.7 |
| n | 6.89 | n | 158.8 |
| o | 7.01 | o | 132.5 |
| p | 3.37 | p | 131.0 |
| r | 3.56 | r | 165.3 |
| s | 4.10 | s | 132.6 |
| t | 7.08 | t | 131.0 |
| Sample | Quantum Yield, % | Quantum Yield After Seven Days, % | Quantum Yield After 30 Days, % |
|---|---|---|---|
| Rhod-OH | 31 | 28 | 22 |
| Dot-1 | 3.4 | 3.2 | 3.0 |
| Dot-2 | 1.1 | 1.1 | 1.0 |
| Dot-3 | 3.9 | 3.6 | 3.5 |
| Dot-4 | 0.9 | 0.9 | 0.9 |
| Dot-1-R | 14.1 | 14.1 | 14.0 |
| Dot-2-R | 17.0 | 16.9 | 16.8 |
| Dot-3-R | 11.2 | 11.2 | 11.0 |
| Dot-4-R | 4.9 | 4.9 | 4.9 |
| Sample | % of OH• Free Radicals Removed | % of O2•− Free Radicals Removed |
|---|---|---|
| Dot-1 | 75 | 78 |
| Dot-2 | 90 | 92 |
| Dot-3 | 88 | 90 |
| Dot-4 | 80 | 83 |
| Dot-1-R | 81 | 83 |
| Dot-2-R | 84 | 87 |
| Dot-3-R | 82 | 85 |
| Dot-4-R | 82 | 84 |
| Sample | Glucose g per 20 mL H2O | Acid, Amount | Reaction Time, h | Temperature, °C |
|---|---|---|---|---|
| Dot-1 | 9 | Formic, 3.0 mL | 12 | 180 |
| Dot-2 | Aspartic, 1.5 g | |||
| Dot-3 | Tartaric, 1.5 g | |||
| Dot-4 | Hydrochloric, 1.5 mL |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janus, Ł.; Radwan-Pragłowska, J.; Piątkowski, M.; Bogdał, D. Smart, Tunable CQDs with Antioxidant Properties for Biomedical Applications—Ecofriendly Synthesis and Characterization. Molecules 2020, 25, 736. https://doi.org/10.3390/molecules25030736
Janus Ł, Radwan-Pragłowska J, Piątkowski M, Bogdał D. Smart, Tunable CQDs with Antioxidant Properties for Biomedical Applications—Ecofriendly Synthesis and Characterization. Molecules. 2020; 25(3):736. https://doi.org/10.3390/molecules25030736
Chicago/Turabian StyleJanus, Łukasz, Julia Radwan-Pragłowska, Marek Piątkowski, and Dariusz Bogdał. 2020. "Smart, Tunable CQDs with Antioxidant Properties for Biomedical Applications—Ecofriendly Synthesis and Characterization" Molecules 25, no. 3: 736. https://doi.org/10.3390/molecules25030736
APA StyleJanus, Ł., Radwan-Pragłowska, J., Piątkowski, M., & Bogdał, D. (2020). Smart, Tunable CQDs with Antioxidant Properties for Biomedical Applications—Ecofriendly Synthesis and Characterization. Molecules, 25(3), 736. https://doi.org/10.3390/molecules25030736