Free-standing Reduced Graphene Oxide/Carbon Nanotube Paper for Flexible Sodium-ion Battery Applications
Abstract
:1. Introduction
2. Experimental
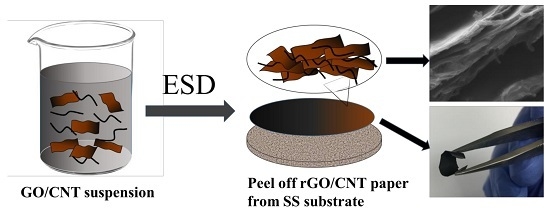
2.1. Fabrication of Freestanding rGO/CNT Paper
2.2. Characterization and Electrochemical Measurements
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Etacheri, V.; Marom, R.; Elazari, R.; Salitra, G.; Aurbach, D. Challenges in the development of advanced Li-ion batteries: A review. Energy Environ. Sci. 2011, 4, 3243–3262. [Google Scholar] [CrossRef]
- Pan, H.; Hu, Y.-S.; Chen, L. Room-temperature stationary sodium-ion batteries for large-scale electric energy storage. Energy Environ. Sci. 2013, 6, 2338. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Kubota, K.; Dahbi, M.; Komaba, S. Research Development on Sodium-Ion Batteries. Chem. Rev. 2014, 114, 11636–11682. [Google Scholar] [CrossRef]
- Slater, M.D.; Kim, N.; Lee, E.; Johnson, C.S. Correction: Sodium-Ion Batteries. Adv. Funct. Mater. 2013, 23, 3255. [Google Scholar] [CrossRef]
- Wang, L.P.; Yu, L.; Wang, X.; Srinivasan, M.; Xu, Z.J. Recent developments in electrode materials for sodium-ion batteries. J. Mater. Chem. A 2015, 3, 9353–9378. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Jia, M.; Cao, B.; Chen, R.; Lv, X.; Tang, R.; Wu, F.; Xu, B. Nitrogen-doped carbon/graphene hybrid anode material for sodium-ion batteries with excellent rate capability. J. Power Sources 2016, 319, 195–201. [Google Scholar] [CrossRef]
- An, H.; Li, Y.; Gao, Y.; Cao, C.; Han, J.; Feng, Y.; Feng, W. Free-standing fluorine and nitrogen co-doped graphene paper as a high-performance electrode for flexible sodium-ion batteries. Carbon 2017, 116, 338–346. [Google Scholar] [CrossRef]
- Kang, H.; Liu, Y.; Cao, K.; Zhao, Y.; Jiao, L.; Wang, Y.; Yuan, H. Update on anode materials for Na-ion batteries. J. Mater. Chem. A 2015, 3, 17899–17913. [Google Scholar] [CrossRef]
- Wang, Y.; Kong, D.; Shi, W.; Liu, B.; Sim, G.J.; Ge, Q.; Yang, H.Y. Ice Templated Free-Standing Hierarchically WS2/CNT-rGO Aerogel for High-Performance Rechargeable Lithium and Sodium Ion Batteries. Adv. Energy Mater. 2016, 6, 1601057. [Google Scholar] [CrossRef]
- Wang, X.W.; Guo, H.-P.; Liang, J.; Zhang, J.-F.; Zhang, B.; Wang, J.-Z.; Luo, W.; Liu, H.K.; Dou, S.X. An Integrated Free-Standing Flexible Electrode with Holey-Structured 2D Bimetallic Phosphide Nanosheets for Sodium-Ion Batteries. Adv. Funct. Mater. 2018, 28, 1801016. [Google Scholar] [CrossRef]
- David, L.; Bhandavat, L.; Singh, G. MoS2/graphene composite paper for sodium-ion battery electrodes. ACS Nano 2014, 8, 1759–1770. [Google Scholar]
- Xie, X.; Makaryan, T.; Zhao, M.; Van Aken, K.L.; Gogotsi, Y.; Wang, G. MoS2 Nanosheets Vertically Aligned on Carbon Paper: A Freestanding Electrode for Highly Reversible Sodium-Ion Batteries. Adv. Energy Mater. 2015, 6, 1502161. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, X.; Mi, L.; Liu, C.; Zhang, J.; Cui, S.; Feng, X.; Cao, Y.; Shen, C. High-Performance Flexible Freestanding Anode with Hierarchical 3D Carbon-Networks/Fe7S8/Graphene for Applicable Sodium-Ion Batteries. Adv. Mater. 2019, 31, 1806664. [Google Scholar] [CrossRef]
- Yan, Y.; Yin, Y.-X.; Guo, Y.; Wan, L.-J. A Sandwich-Like Hierarchically Porous Carbon/Graphene Composite as a High-Performance Anode Material for Sodium-Ion Batteries. Adv. Energy Mater. 2014, 4, 1301584. [Google Scholar] [CrossRef]
- Li, X.; Hu, X.; Zhou, L.; Wen, R.; Xu, X.; Chou, S.; Chen, L.; Cao, A.-M.; Dou, S. A S/N-doped high-capacity mesoporous carbon anode for Na-ion batteries. J. Mater. Chem. A 2019, 7, 11976–11984. [Google Scholar] [CrossRef]
- Sun, N.; Guan, Y.; Liu, Y.-T.; Zhu, Q.; Shen, J.; Liu, H.; Zhou, S.; Xu, B. Facile synthesis of free-standing, flexible hard carbon anode for high-performance sodium ion batteries using graphene as a multi-functional binder. Carbon 2018, 137, 475–483. [Google Scholar] [CrossRef]
- Jin, J.; Shi, Z.-Q.; Wang, C.-Y. Electrochemical Performance of Electrospun carbon nanofibers as free-standing and binder-free anodes for Sodium-Ion and Lithium-Ion Batteries. Electrochim. Acta 2014, 141, 302–310. [Google Scholar] [CrossRef]
- Wang, S.; Xia, L.; Yu, L.; Zhang, L.; Wang, H.; Lou, X.W. Free-Standing Nitrogen-Doped Carbon Nanofiber Films: Integrated Electrodes for Sodium-Ion Batteries with Ultralong Cycle Life and Superior Rate Capability. Adv. Energy Mater. 2016, 6, 1502217. [Google Scholar] [CrossRef]
- Xu, J.; Wang, M.; Wickramaratne, N.P.; Jaroniec, M.; Dou, S.X.; Dai, L. High-Performance Sodium Ion Batteries Based on a 3D Anode from Nitrogen-Doped Graphene Foams. Adv. Mater. 2015, 27, 2042–2048. [Google Scholar] [CrossRef]
- Kim, S.; Li, X.; Sang, L.; Yun, Y.S.; Nuzzo, R.; Gewirth, A.A.; Braun, P.V. High Energy Density CNT/NaI Composite Cathodes for Sodium-Ion Batteries. Adv. Mater. Interfaces 2018, 5, 1801342. [Google Scholar] [CrossRef]
- Xie, X.; Kretschmer, K.; Zhang, J.; Sun, B.; Su, D.; Wang, G. Sn@CNT nanopillars grown perpendicularly on carbon paper: A novel free-standing anode for sodium ion batteries. Nano Energy 2015, 13, 208–217. [Google Scholar] [CrossRef]
- Jin, T.; Han, Q.; Jiao, L. Binder-Free Electrodes for Advanced Sodium-Ion Batteries. Adv. Mater. 2019, 32, e1806304. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Lee, K.T.; Hitz, G.T.; Han, X.; Li, Y.; Wan, J.; Lacey, S.; Cresce, A.V.W.; Xu, K.; Wachsman, E. Free-standing Na2/3Fe1/2Mn1/2O2@graphene film for a sodium-ion battery cathode. ACS Appl. Mater. Interfaces 2014, 6, 4242–4247. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Song, M.-S.; Kong, B.; Cui, Y. Flexible and Stretchable Energy Storage: Recent Advances and Future Perspectives. Adv. Mater. 2016, 29, 1603436. [Google Scholar] [CrossRef] [PubMed]
- Tao, T.; Lu, S.; Chen, Y. A Review of Advanced Flexible Lithium-Ion Batteries. Adv. Mater. Technol. 2018, 3, 1700375. [Google Scholar] [CrossRef]
- Wang, H.; Li, W.; Liu, D.; Feng, X.; Wang, J.; Yang, X.; Zhang, X.; Zhu, Y.; Zhang, Y. Flexible Electrodes for Sodium-Ion Batteries: Recent Progress and Perspectives. Adv. Mater. 2017, 29, 1703012. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Li, W.; Zeng, L.; Yang, Z.; Gu, L.; Wang, J.; Liu, X.; Cheng, J.; Yu, Y. Free-standing and binder-free sodium-ion electrodes with ultralong cycle life and high rate performance based on porous carbon nanofibers. Nanoscale 2014, 6, 693–698. [Google Scholar] [CrossRef]
- Fu, L.; Tang, K.; Song, K.; Van Aken, P.A.; Yu, Y.; Maier, J. Nitrogen doped porous carbon fibres as anode materials for sodium ion batteries with excellent rate performance. Nanoscale 2014, 6, 1384–1389. [Google Scholar] [CrossRef]
- Jin, J.; Yu, B.-J.; Shi, Z.-Q.; Wang, C.-Y.; Chong, C.-B. Lignin-based electrospun carbon nanofibrous webs as free-standing and binder-free electrodes for sodium ion batteries. J. Power Sources 2014, 272, 800–807. [Google Scholar] [CrossRef]
- Abouimrane, A.; Compton, O.C.; Amine, K.; Nguyen, S. Non-Annealed Graphene Paper as a Binder-Free Anode for Lithium-Ion Batteries. J. Phys. Chem. C 2010, 114, 12800–12804. [Google Scholar] [CrossRef]
- Hao, Y.; Li, X.; Sun, X.; Wang, C. Nitrogen-Doped Graphene Nanosheets/S Composites as Cathode in Room-Temperature Sodium-Sulfur Batteries. ChemistrySelect 2017, 2, 9425–9432. [Google Scholar] [CrossRef]
- Ruan, J.; Yuan, T.; Pang, Y.; Luo, S.; Peng, C.; Yang, J.; Zheng, S. Nitrogen and sulfur dual-doped carbon films as flexible free-standing anodes for Li-ion and Na-ion batteries. Carbon 2018, 126, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Zhang, L.; Chen, H.; Wang, J.; Ding, L.-X.; Wang, S.; Ashman, P.; Wang, H. Graphene-based nitrogen-doped carbon sandwich nanosheets: A new capacitive process controlled anode material for high-performance sodium-ion batteries. J. Mater. Chem. A 2016, 4, 8630–8635. [Google Scholar] [CrossRef]
- Yuan, J.; Chen, C.; Hao, Y.; Zhang, X.; Agrawal, R.; Zhao, W.; Wang, C.; Yu, H.; Zhu, X.; Yu, Y.; et al. Fabrication of three-dimensional porous ZnMn2O4 thin films on Ni foams through electrostatic spray deposition for high-performance lithium-ion battery anodes. J. Alloy. Compd. 2017, 696, 1174–1179. [Google Scholar] [CrossRef] [Green Version]
- Yuan, J.; Chen, C.; Hao, Y.; Zhang, X.; Gao, S.; Agrawal, R.; Wang, C.; Xiong, Z.; Yu, H.; Xie, Y. A facile synthetic strategy to three-dimensional porous ZnCo2O4 thin films on Ni foams for high-performance lithium-ion battery anodes. J. Electroanal. Chem. 2017, 787, 158–162. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Wang, C. Engineering nanostructured anodes via electrostatic spray deposition for high performance lithium ion battery application. J. Mater. Chem. A 2013, 1, 165–182. [Google Scholar] [CrossRef]
- Zhu, C.; Fu, Y.; Yu, Y. Designed Nanoarchitectures by Electrostatic Spray Deposition for Energy Storage. Adv. Mater. 2018, 31, 1803408. [Google Scholar] [CrossRef] [Green Version]
- Beidaghi, M.; Wang, C. Micro-Supercapacitors Based on Interdigital Electrodes of Reduced Graphene Oxide and Carbon Nanotube Composites with Ultrahigh Power Handling Performance. Adv. Funct. Mater. 2012, 22, 4501–4510. [Google Scholar] [CrossRef]
- Adelowo, E.; Baboukani, A.R.; Chen, C.; Wang, C. Electrostatically Sprayed Reduced Graphene Oxide-Carbon Nanotubes Electrodes for Lithium-Ion Capacitors. C—J. Carbon Res. 2018, 4, 31. [Google Scholar] [CrossRef] [Green Version]
- Manickam, S.; Muthoosamy, K.; Bai, R.G.; Abubakar, I.B.; Sudheer, S.M.; Hongngee, L.; Hwei-San, L.; Nayming, H.; Chia, C.H.; Lim, H.N.; et al. Exceedingly biocompatible and thin-layered reduced graphene oxide nanosheets using an eco-friendly mushroom extract strategy. Int. J. Nanomed. 2015, 10, 1505–1519. [Google Scholar] [CrossRef] [Green Version]
- Mishra, S.K.; Tripathi, S.N.; Choudhary, V.; Gupta, B. Surface Plasmon Resonance-Based Fiber Optic Methane Gas Sensor Utilizing Graphene-Carbon Nanotubes-Poly(Methyl Methacrylate) Hybrid Nanocomposite. Plasmon 2015, 10, 1147–1157. [Google Scholar] [CrossRef]
- Wang, K.; Pang, J.; Li, L.; Zhou, S.; Li, Y.; Zhang, T. Synthesis of hydrophobic carbon nanotubes/reduced graphene oxide composite films by flash light irradiation. Front. Chem. Sci. Eng. 2018, 12, 376–382. [Google Scholar] [CrossRef]
- Ali, G.; Mehmood, A.; Ha, H.Y.; Kim, J.; Chung, K.Y. Reduced graphene oxide as a stable and high-capacity cathode material for Na-ion batteries. Sci. Rep. 2017, 7, 40910. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Zhou, J.; Cao, X.; Liu, S.; Cai, Y.; Wang, L.; Pan, A.; Liang, S. Graphene oxide templated nitrogen-doped carbon nanosheets with superior rate capability for sodium ion batteries. Carbon 2017, 122, 82–91. [Google Scholar] [CrossRef]
- David, L.; Singh, G. Reduced Graphene Oxide Paper Electrode: Opposing Effect of Thermal Annealing on Li and Na Cyclability. J. Phys. Chem. C 2014, 118, 28401–28408. [Google Scholar] [CrossRef]
- Hu, L.; Shang, C.; Huang, L.; Wang, X.; Zhou, G. Cu3Ge coated by nitrogen-doped carbon nanorods as advanced sodium-ion battery anodes. Ionics 2019, 26, 719–726. [Google Scholar] [CrossRef]
- Shang, C.; Hu, L.; Fu, L.; Huang, L.; Xue, B.; Wang, X.; Shui, L.; Zhou, G. Improving lithium storage capability of ternary Sn-based sulfides by enhancing inactive/active element ratio. Solid State Ionics 2019, 337, 47–55. [Google Scholar] [CrossRef]
- Shang, C.; Hu, L.; Lin, Q.; Fu, X.; Wang, X.; Zhou, G. Integration of NaV6O15 · nH2O nanowires and rGO as cathode materials for efficient sodium storage. Appl. Surf. Sci. 2019, 494, 458–464. [Google Scholar] [CrossRef]
- Hao, Y.; Chen, C.; Yang, X.; Xiao, G.; Zou, B.; Yang, J.; Wang, C. Studies on intrinsic phase-dependent electrochemical properties of MnS nanocrystals as anodes for lithium-ion batteries. J. Power Sources 2017, 338, 9–16. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of the compounds are not available from the authors. |





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, Y.; Wang, C. Free-standing Reduced Graphene Oxide/Carbon Nanotube Paper for Flexible Sodium-ion Battery Applications. Molecules 2020, 25, 1014. https://doi.org/10.3390/molecules25041014
Hao Y, Wang C. Free-standing Reduced Graphene Oxide/Carbon Nanotube Paper for Flexible Sodium-ion Battery Applications. Molecules. 2020; 25(4):1014. https://doi.org/10.3390/molecules25041014
Chicago/Turabian StyleHao, Yong, and Chunlei Wang. 2020. "Free-standing Reduced Graphene Oxide/Carbon Nanotube Paper for Flexible Sodium-ion Battery Applications" Molecules 25, no. 4: 1014. https://doi.org/10.3390/molecules25041014
APA StyleHao, Y., & Wang, C. (2020). Free-standing Reduced Graphene Oxide/Carbon Nanotube Paper for Flexible Sodium-ion Battery Applications. Molecules, 25(4), 1014. https://doi.org/10.3390/molecules25041014