Structure-Activity Relationship between Thiol Group-Trapping Ability of Morphinan Compounds with a Michael Acceptor and Anti-Plasmodium falciparum Activities
Abstract
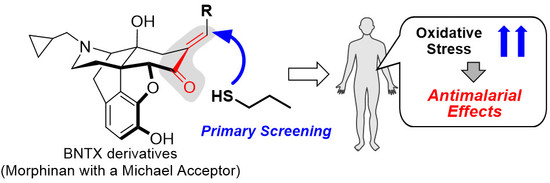
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Experimental Data
3.2. Antimalarial Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Malaria. Available online: https://www.who.int/news-room/fact-sheets/detail/malaria (accessed on 4 October 2019).
- Beare, N.A.V.; Lewallen, S.; Taylor, T.E.; Molyneux, M.E. Redefining cerebral malaria by including malaria retinopathy. Future Microbiol. 2011, 6, 349–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartoloni, A.; Zammarchi, L. Clinical aspects of uncomplicated and severe malaria. Mediterr. J. Hematol. Infect. Dis. 2012, 4, e2012026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, W.R.J.; Hanson, J.; Turner, G.D.H.; White, N.J.; Dondorp, A.M. Respiratory manifestations of malaria. Chest 2012, 142, 492–505. [Google Scholar] [CrossRef] [PubMed]
- Wongsrichanalai, C.; Meshnick, S.R. Declining artesunate-mefloquine efficacy against falciparum malaria on the Cambodia-Thailand border. Emerg. Infect. Dis. 2008, 14, 716–719. [Google Scholar] [CrossRef] [PubMed]
- Ashley, E.A.; Dhorda, M.; Fairhurst, R.M.; Amaratunga, C.; Lim, P.; Suon, S.; Sreng, S.; Anderson, J.M.; Mao, S.; Sam, B.; et al. Spread of artemisinin resistance in Plasmodium falciparum malaria. N. Engl. J. Med. 2014, 371, 411–423. [Google Scholar] [CrossRef] [Green Version]
- Miyata, Y.; Fujii, H.; Osa, Y.; Kobayashi, S.; Takeuchi, T.; Nagase, H. Opioid δ1 receptor antagonist 7-benzylidenenaltrexone as an effective resistance reverser for chloroquine-resistant Plasmodium chabaudi. Bioorg. Med. Chem. Lett. 2011, 21, 4710–4712. [Google Scholar] [CrossRef]
- Miyata, Y.; Fujii, H.; Uenohara, Y.; Kobayashi, S.; Takenouchi, T.; Nagase, H. Investigation of 7-benzylidenenaltrexone derivatives as resistance reverser for chloroquine-resistant Plasmodium chabaudi. Bioorg. Med. Chem. Lett. 2012, 22, 5174–5176. [Google Scholar] [CrossRef]
- Asahi, H.; Inoue, S.-I.; Niikura, M.; Kunigo, K.; Suzuki, Y.; Kobayashi, F.; Sendo, F. Profiling molecular factors associated with pyknosis and developmental arrest induced by an opioid receptor antagonist and dihydroarthemisinin in Plasmodium falciparum. PLoS ONE 2017, 12, e0184874. [Google Scholar] [CrossRef] [Green Version]
- Kutsumura, N.; Nakajima, R.; Koyama, Y.; Miyata, Y.; Saitoh, T.; Yamamoto, N.; Iwata, S.; Fujii, H.; Nagase, H. Investigation of 7-benzylidenenaltrexone derivatives as a novel structural antitrichomonal lead compound. Bioorg. Med. Chem. Lett. 2015, 25, 4890–4892. [Google Scholar] [CrossRef]
- Kutsumura, N.; Koyama, Y.; Nagumo, Y.; Nakajima, R.; Miyata, Y.; Yamamoto, N.; Saitoh, T.; Yoshida, N.; Iwata, S.; Nagase, H. Antitrichomonal activity of δ opioid receptor antagonists, 7-benzylidenenaltrexone derivatives. Bioorg. Med. Chem. 2017, 25, 4375–4383. [Google Scholar] [CrossRef]
- Dubois, V.L.; Platel, D.F.N.; Pauly, G.; Tribouleyduret, J. Plasmodium berghei: Implication of intracellular glutathione and its related enzyme in chloroquine resistance in vivo. Exp. Parasitol. 1995, 81, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Ginsburg, H.; Famin, O.; Zhang, J.; Krugliak, M. Inhibition of glutathione-dependent degradation of heme by chloroquine and amodiaquine as a possible basis for their antimalarial mode of action. Biochem. Pharmacol. 1998, 56, 1305–1313. [Google Scholar] [CrossRef]
- Famin, O.; Krugliak, M.; Ginsburg, H. Kinetics of inhibition of glutathione-mediated degradation of ferriprotoporphyrin IX by antimalarial drugs. Biochem. Pharmacol. 1999, 58, 59–68. [Google Scholar] [CrossRef]
- Ellis, J.E.; Yarlett, N.; Cole, D.; Humphreys, M.J.; Lloyd, D. Antioxidant defences in the microaerophilic protozoan Trichomonas vaginalis: Comparison of metronidazole-resistant and sensitive strains. Microbiology 1994, 140, 2489–2494. [Google Scholar] [CrossRef] [Green Version]
- Coombs, G.H.; Westrop, G.D.; Suchan, P.; Puzova, G.; Hirt, R.P.; Embley, T.M.; Mottram, J.C.; Müller, S. The amitochondriate eukaryote Trichomonas vaginalis contains a divergent thioredoxin-linked peroxiredoxin antioxidant system. J. Biol. Chem. 2004, 279, 5249–5256. [Google Scholar] [CrossRef] [Green Version]
- Pal, C.; Bandyopadhyay, U. Redox-active antiparasitic drugs. Antiox. Redox Signal. 2012, 17, 555–587. [Google Scholar] [CrossRef]
- Beltrán, N.C.; Horváthová, L.; Jedelský, P.L.; Šedinová, M.; Rada, P.; Marcinčiková, M.; Hrdý, I.; Tachezy, J. Iron-induced changes in the proteome of Trichomonas vaginalis hydrogenosomes. PLoS ONE 2013, 8, e65148. [Google Scholar] [CrossRef] [Green Version]
- Puente-Rivera, J.; Ramón-Luing, L.Á.; Figueroa-Angulo, E.E.; Ortega-López, J.; Arroyo, R. Trichocystatin-2(TC-2): An endogenous inhibitor of cysteine proteinases in Trichomonas vaginalis is associated with TvCP39. Int. J. Biochem. Cell Biol. 2014, 54, 255–265. [Google Scholar] [CrossRef]
- Nagase, H.; Hayakawa, J.; Kawamura, K.; Kawai, K.; Takezawa, Y.; Matsuura, H.; Tajima, C.; Endo, T. Discovery of a structurally novel opioid κ-agonist derived from 4,5-epoxymorphinan. Chem. Pharm. Bull. 1998, 46, 366–369. [Google Scholar] [CrossRef] [Green Version]
- Otoguro, K.; Kohana, A.; Manabe, C.; Ishiyama, A.; Ui, H.; Shiomi, K.; Yamada, H.; Omura, S. Potent antimalarial activities of polyether antibiotic, X-206. J. Antibiot. 2001, 54, 658–663. [Google Scholar] [CrossRef] [Green Version]
- Van’t Erve, T.J.; Wagner, B.A.; Ryckman, K.K.; Raife, T.J.; Buettner, G.R. The concentration of glutathione in human erythrocytes is a heritable trait. Free Radic. Biol. Med. 2013, 65, 742–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atamna, H.; Ginsburg, H. The malaria parasite supplies glutathione to its host cell–Investigation of glutathione transport and metabolism in human erythrocytes infected with Plasmodium falciparum. Eur. J. Biochem. 1997, 250, 670–679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, T.; Johann, L.; Jannack, B.; Brückner, M.; Lanfranchi, D.A.; Bauer, H.; Sanchez, C.; Yardley, V.; Deregnaucourt, C.; Schrével, J.; et al. Glutathione reductase-catalyzed cascade of redox reactions to bioactive potent antimalarial 1,4-naphthoquinones–A new strategy to combat malarial parasites. J. Am. Chem. Soc. 2011, 133, 11557–11571. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Sabnis, Y.; Zhao, Z.; Zhang, T.; Buhrlage, S.J.; Jones, L.H.; Gray, N.S. Developing irreversible inhibitors of the protein kinase cysteinome. Chem. Biol. 2013, 20, 146–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanning, B.R.; Whitby, L.R.; Dix, M.M.; Douhan, J.; Gilbert, A.M.; Hett, E.C.; Johnson, T.O.; Joslyn, C.; Kath, J.C.; Niessen, S.; et al. A road map to evaluate the proteome-wide selectivity of covalent kinase inhibitors. Nat. Chem. Biol. 2014, 10, 760–767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goedken, E.R.; Argiriadi, M.A.; Banach, D.L.; Fiamengo, B.A.; Foley, S.E.; Frank, K.E.; George, J.S.; Harris, C.M.; Hobson, A.D.; Ihle, D.C.; et al. Tricyclic covalent inhibitors selectively target Jak3 through an active site thiol. J. Biol. Chem. 2015, 290, 4573–4589. [Google Scholar] [CrossRef] [Green Version]
- Jackson, P.A.; Widen, J.C.; Harki, D.A.; Brummond, K.M. Covalent modifiers: A chemical perspective on the reactivity of α,β-unsaturated carbonyls with thiols via hetero-Michael addition reactions. J. Med. Chem. 2017, 60, 839–885. [Google Scholar] [CrossRef]
- Shindo, N.; Fuchida, H.; Sato, M.; Watari, K.; Shibata, T.; Kuwata, K.; Miura, C.; Okamoto, K.; Hatsuyama, Y.; Tokunaga, K.; et al. Selective and reversible modification of kinase cysteines with chlorofluoroacetamides. Nat. Chem. Biol. 2019, 15, 250–258. [Google Scholar] [CrossRef]
- Ohkawa, S.; Portoghese, P.S. 7-Arylidenenaltrexones as selective δ1 opioid receptor antagonists. J. Med. Chem. 1998, 41, 4177–4180. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |





| Compound | IC50 (μM) | Compound | IC50 (μM) | ||
|---|---|---|---|---|---|
| K1 a | FCR3 b | K1a | FCR3 b | ||
| Artemisinin | 0.03 | 0.04 | 10d | 7.33 | 6.38 |
| Chloroquine | 0.57 | 0.07 | 11c | 2.60 | 2.50 |
| BNTX (1) c | 4.08 | 3.26 | 12d | 3.93 | 4.66 |
| 2d | 4.31 | 3.94 | 13d | 7.89 | 9.43 |
| 3d | 3.60 | 2.86 | 14d | 2.08 | 1.95 |
| 4d | 2.82 | 2.67 | 15d | 7.37 | 7.16 |
| 5d | 3.07 | 2.76 | 16d | 7.58 | 6.61 |
| 6d | 2.78 | 2.24 | 17d | >20.1 | N/A |
| 7d | 3.16 | 2.38 | 18d | 18.3 | 15.0 |
| 8d | 3.04 | 2.24 | 19d | 15.6 | 10.3 |
| 9d | 2.99 | 2.13 | 20d | 19.8 | >21.5 |
| Compound | Residual Rate (%) | Compound | Residual Rate (%) | ||
|---|---|---|---|---|---|
| 1 day | 2 days | 1 day | 2 days | ||
| 1c | 40.2 | 38.7 b | 11a | 35.1 | 14.0 |
| 2c | 36.0 | 34.1 b | 12c | 54.9 | 53.2 |
| 3c | 70.0 | 56.0 | 13c | 45.8 | 38.6 |
| 4c | 46.4 | 36.3 | 14c | 25.3 | 16.2 |
| 5c | 41.8 | 31.2 | 15c | 6.8 | 6.0 |
| 6c | 51.3 | 40.1 | 16c | 79.0 | 66.8 |
| 7c | 24.6 | 17.7 | 17c | 90.0 | 88.3 |
| 8c | 15.6 | 10.3 | 18c | 82.6 | 80.2 |
| 9c | 6.7 | 6.3 | 19c | 90.9 | 83.8 |
| 10c | 36.6 | 24.8 | 20c | 97.9 | 89.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kutsumura, N.; Koyama, Y.; Saitoh, T.; Yamamoto, N.; Nagumo, Y.; Miyata, Y.; Hokari, R.; Ishiyama, A.; Iwatsuki, M.; Otoguro, K.; et al. Structure-Activity Relationship between Thiol Group-Trapping Ability of Morphinan Compounds with a Michael Acceptor and Anti-Plasmodium falciparum Activities. Molecules 2020, 25, 1112. https://doi.org/10.3390/molecules25051112
Kutsumura N, Koyama Y, Saitoh T, Yamamoto N, Nagumo Y, Miyata Y, Hokari R, Ishiyama A, Iwatsuki M, Otoguro K, et al. Structure-Activity Relationship between Thiol Group-Trapping Ability of Morphinan Compounds with a Michael Acceptor and Anti-Plasmodium falciparum Activities. Molecules. 2020; 25(5):1112. https://doi.org/10.3390/molecules25051112
Chicago/Turabian StyleKutsumura, Noriki, Yasuaki Koyama, Tsuyoshi Saitoh, Naoshi Yamamoto, Yasuyuki Nagumo, Yoshiyuki Miyata, Rei Hokari, Aki Ishiyama, Masato Iwatsuki, Kazuhiko Otoguro, and et al. 2020. "Structure-Activity Relationship between Thiol Group-Trapping Ability of Morphinan Compounds with a Michael Acceptor and Anti-Plasmodium falciparum Activities" Molecules 25, no. 5: 1112. https://doi.org/10.3390/molecules25051112
APA StyleKutsumura, N., Koyama, Y., Saitoh, T., Yamamoto, N., Nagumo, Y., Miyata, Y., Hokari, R., Ishiyama, A., Iwatsuki, M., Otoguro, K., Ōmura, S., & Nagase, H. (2020). Structure-Activity Relationship between Thiol Group-Trapping Ability of Morphinan Compounds with a Michael Acceptor and Anti-Plasmodium falciparum Activities. Molecules, 25(5), 1112. https://doi.org/10.3390/molecules25051112