In Vitro and In Vivo Characterization of Dibenzothiophene Derivatives [125I]Iodo-ASEM and [18F]ASEM as Radiotracers of Homo- and Heteromeric α7 Nicotinic Acetylcholine Receptors
Abstract
:1. Introduction
2. Results
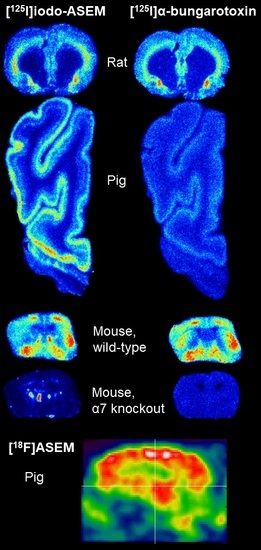
2.1. In Vitro Autoradiography
2.2. In Vivo PET Imaging in the Pig Using [18F]ASEM
3. Discussion
4. Materials and Methods
4.1. Compounds and Radioligands
4.2. Tissue Origin and Sectioning for In Vitro Autoradiography
4.3. In Vitro Autoradiography with [125I]Iodo-ASEM
4.4. In Vitro Autoradiography with [125I]α-bungarotoxin
4.5. Saturation Binding and Kinetic Analysis Using In Vitro Autoradiography
4.6. Autoradiographic Image Acquisition and Analysis
4.7. Radiosynthesis of [18F]ASEM
4.8. In Vivo Imaging in the Pig
4.8.1. Animal Procedures
4.8.2. PET Scanning
4.8.3. Blood Sampling
4.8.4. Metabolite Analysis
4.8.5. Determination of Free Fraction
4.8.6. Reconstruction and Pre-Processing of PET Data
4.8.7. Kinetic Modelling of PET Data
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Marutle, A.; Zhang, X.; Court, J.; Piggott, M.; Johnson, M.; Perry, R.; Perry, E.; Nordberg, A. Laminar distribution of nicotinic receptor subtypes in cortical regions in schizophrenia. J. Chem. Neuroanat. 2001, 22, 115–126. [Google Scholar] [CrossRef]
- Kulak, J.M.; Carroll, F.I.; Schneider, J.S. [125I]Iodomethyllycaconitine binds to alpha7 nicotinic acetylcholine receptors in monkey brain. Eur. J. Neurosci. 2006, 23, 2604–2610. [Google Scholar] [CrossRef] [PubMed]
- Whiteaker, P.; Davies, A.R.; Marks, M.J.; Blagbrough, I.S.; Potter, B.V.; Wolstenholme, A.J.; Collins, A.C.; Wonnacott, S. An autoradiographic study of the distribution of binding sites for the novel alpha7-selective nicotinic radioligand [3H]-methyllycaconitine in the mouse brain. Eur. J. Neurosci. 1999, 11, 2689–2696. [Google Scholar] [CrossRef] [PubMed]
- Quik, M.; Choremis, J.; Komourian, J.; Lukas, R.J.; Puchacz, E. Similarity between rat brain nicotinic alpha-bungarotoxin receptors and stably expressed alpha-bungarotoxin binding sites. J. Neurochem. 1996, 67, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Lendvai, B.; Kassai, F.; Szajli, A.; Nemethy, Z. alpha7 nicotinic acetylcholine receptors and their role in cognition. Brain Res. Bull. 2013, 93, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Picciotto, M.R.; Lewis, A.S.; van Schalkwyk, G.I.; Mineur, Y.S. Mood and anxiety regulation by nicotinic acetylcholine receptors: A potential pathway to modulate aggression and related behavioral states. Neuropharmacology 2015, 96, 235–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albuquerque, E.X.; Pereira, E.F.; Alkondon, M.; Rogers, S.W. Mammalian nicotinic acetylcholine receptors: From structure to function. Physiol. Rev. 2009, 89, 73–120. [Google Scholar] [CrossRef] [Green Version]
- Dani, J.A.; Bertrand, D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu Rev Pharm. Toxicol 2007, 47, 699–729. [Google Scholar] [CrossRef]
- Maurer, S.V.; Williams, C.L. The Cholinergic System Modulates Memory and Hippocampal Plasticity via Its Interactions with Non-Neuronal Cells. Front Immunol. 2017, 8, 1489. [Google Scholar] [CrossRef] [Green Version]
- Bosmans, G.; Shimizu Bassi, G.; Florens, M.; Gonzalez-Dominguez, E.; Matteoli, G.; Boeckxstaens, G.E. Cholinergic Modulation of Type 2 Immune Responses. Front Immunol. 2017, 8, 1873. [Google Scholar] [CrossRef] [Green Version]
- Freedman, R.; Hall, M.; Adler, L.E.; Leonard, S. Evidence in postmortem brain tissue for decreased numbers of hippocampal nicotinic receptors in schizophrenia. Biol. Psychiatry 1995, 38, 22–33. [Google Scholar] [CrossRef]
- Guillozet-Bongaarts, A.L.; Hyde, T.M.; Dalley, R.A.; Hawrylycz, M.J.; Henry, A.; Hof, P.R.; Hohmann, J.; Jones, A.R.; Kuan, C.L.; Royall, J.; et al. Altered gene expression in the dorsolateral prefrontal cortex of individuals with schizophrenia. Mol. Psychiatry 2014, 19, 478–485. [Google Scholar] [CrossRef] [Green Version]
- Kunii, Y.; Zhang, W.; Xu, Q.; Hyde, T.M.; McFadden, W.; Shin, J.H.; Deep-Soboslay, A.; Ye, T.; Li, C.; Kleinman, J.E.; et al. CHRNA7 and CHRFAM7A mRNAs: Co-Localized and Their Expression Levels Altered in the Postmortem Dorsolateral Prefrontal Cortex in Major Psychiatric Disorders. Am. J. Psychiatry 2015. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, M.S.; Weyn, A.; Mikkelsen, J.D. Hippocampal alpha7 nicotinic acetylcholine receptor levels in patients with schizophrenia, bipolar disorder, or major depressive disorder. Bipolar Disord. 2011, 13, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Sugaya, K.; Giacobini, E.; Chiappinelli, V.A. Nicotinic acetylcholine receptor subtypes in human frontal cortex: Changes in Alzheimer’s disease. J. Neurosci. Res. 1990, 27, 349–359. [Google Scholar] [CrossRef]
- Araud, T.; Graw, S.; Berger, R.; Lee, M.; Neveu, E.; Bertrand, D.; Leonard, S. The chimeric gene CHRFAM7A, a partial duplication of the CHRNA7 gene, is a dominant negative regulator of alpha7*nAChR function. Biochem. Pharmacol. 2011, 82, 904–914. [Google Scholar] [CrossRef] [Green Version]
- Gillentine, M.A.; Lozoya, R.; Yin, J.; Grochowski, C.M.; White, J.J.; Schaaf, C.P.; Calarge, C.A. CHRNA7 copy number gains are enriched in adolescents with major depressive and anxiety disorders. J. Affect. Disord. 2018, 239, 247–252. [Google Scholar] [CrossRef]
- Sinkus, M.L.; Graw, S.; Freedman, R.; Ross, R.G.; Lester, H.A.; Leonard, S. The human CHRNA7 and CHRFAM7A genes: A review of the genetics, regulation, and function. Neuropharmacology 2015. [Google Scholar] [CrossRef] [Green Version]
- Gillentine, M.A.; Berry, L.N.; Goin-Kochel, R.P.; Ali, M.A.; Ge, J.; Guffey, D.; Rosenfeld, J.A.; Hannig, V.; Bader, P.; Proud, M.; et al. The Cognitive and Behavioral Phenotypes of Individuals with CHRNA7 Duplications. J. Autism Dev. Disord. 2017, 47, 549–562. [Google Scholar] [CrossRef] [Green Version]
- Hua, S.; Ek, C.J.; Mallard, C.; Johansson, M.E. Perinatal hypoxia-ischemia reduces alpha 7 nicotinic receptor expression and selective alpha 7 nicotinic receptor stimulation suppresses inflammation and promotes microglial Mox phenotype. Biomed Res. Int. 2014, 2014, 718769. [Google Scholar] [CrossRef] [Green Version]
- Han, Z.; Li, L.; Wang, L.; Degos, V.; Maze, M.; Su, H. Alpha-7 nicotinic acetylcholine receptor agonist treatment reduces neuroinflammation, oxidative stress, and brain injury in mice with ischemic stroke and bone fracture. J. Neurochem. 2014, 131, 498–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dash, P.K.; Zhao, J.; Kobori, N.; Redell, J.B.; Hylin, M.J.; Hood, K.N.; Moore, A.N. Activation of Alpha 7 Cholinergic Nicotinic Receptors Reduce Blood-Brain Barrier Permeability following Experimental Traumatic Brain Injury. J. Neurosci. 2016, 36, 2809–2818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mavropoulos, S.A.; Khan, N.S.; Levy, A.C.J.; Faliks, B.T.; Sison, C.P.; Pavlov, V.A.; Zhang, Y.; Ojamaa, K. Nicotinic acetylcholine receptor-mediated protection of the rat heart exposed to ischemia reperfusion. Mol. Med. 2017, 23. [Google Scholar] [CrossRef] [PubMed]
- Gatson, J.W.; Simpkins, J.W.; Uteshev, V.V. High therapeutic potential of positive allosteric modulation of alpha7 nAChRs in a rat model of traumatic brain injury: Proof-of-concept. Brain Res. Bull. 2015, 112, 35–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pike, V.W. Considerations in the Development of Reversibly Binding PET Radioligands for Brain Imaging. Nicotin4 2016, 23, 1818–1869. [Google Scholar]
- Gao, Y.; Kellar, K.J.; Yasuda, R.P.; Tran, T.; Xiao, Y.; Dannals, R.F.; Horti, A.G. Derivatives of dibenzothiophene for positron emission tomography imaging of alpha7-nicotinic acetylcholine receptors. J. Med. Chem. 2013, 56, 7574–7589. [Google Scholar] [CrossRef] [Green Version]
- Toyohara, J.; Hashimoto, K. α7 Nicotinic Receptor Agonists: Potential Therapeutic Drugs for Treatment of Cognitive Impairments in Schizophrenia and Alzheimer’s Disease. Open Med. Chem. J. 2010, 4, 37–56. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, K.; Nishiyama, S.; Ohba, H.; Matsuo, M.; Kobashi, T.; Takahagi, M.; Iyo, M.; Kitashoji, T.; Tsukada, H. [11C]CHIBA-1001 as a novel PET ligand for α7 nicotinic receptors in the brain: A PET study in conscious monkeys. PLoS ONE 2008, 3, e3231. [Google Scholar] [CrossRef]
- Rötering, S.; Scheunemann, M.; Fischer, S.; Hiller, A.; Peters, D.; Deuther-Conrad, W.; Brust, P. Radiosynthesis and first evaluation in mice of [(18)F]NS14490 for molecular imaging of alpha7 nicotinic acetylcholine receptors. Bioorganic Med. Chem. 2013, 21, 2635–2642. [Google Scholar]
- Kim, S.W.; Ding, Y.S.; Alexoff, D.; Patel, V.; Logan, J.; Lin, K.S.; Shea, C.; Muench, L.; Xu, Y.; Carter, P.; et al. Synthesis and positron emission tomography studies of C-11-labeled isotopomers and metabolites of GTS-21, a partial alpha7 nicotinic cholinergic agonist drug. Nucl. Med. Biol. 2007, 34, 541–551. [Google Scholar] [CrossRef] [Green Version]
- Ettrup, A.; Mikkelsen, J.D.; Lehel, S.; Madsen, J.; Nielsen, E.O.; Palner, M.; Timmermann, D.B.; Peters, D.; Knudsen, G.M. 11C-NS14492 as a novel PET radioligand for imaging cerebral alpha7 nicotinic acetylcholine receptors: In vivo evaluation and drug occupancy measurements. J. Nucl. Med. 2011, 52, 1449–1456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deuther-Conrad, W.; Fischer, S.; Hiller, A.; Becker, G.; Cumming, P.; Xiong, G.; Funke, U.; Sabri, O.; Peters, D.; Brust, P. Assessment of alpha7 nicotinic acetylcholine receptor availability in juvenile pig brain with [(18)F]NS10743. Eur. J. Nucl. Med. Mol. Imaging 2011. [Google Scholar] [CrossRef] [PubMed]
- Ouach, A.; Vercouillie, J.; Bertrand, E.; Rodrigues, N.; Pin, F.; Serriere, S.; Boiaryna, L.; Chartier, A.; Percina, N.; Tangpong, P.; et al. Bis(het)aryl-1,2,3-triazole quinuclidines as alpha7 nicotinic acetylcholine receptor ligands: Synthesis, structure affinity relationships, agonism activity, [(18)F]-radiolabeling and PET study in rats. Eur. J. Med. Chem. 2019, 179, 449–469. [Google Scholar] [CrossRef] [PubMed]
- Huan, W.; Aiqin, W.; Jianping, L.; Qianqian, X.; Xia, L.; Lei, Y.; Yu, F.; Huabei, Z. Radiosynthesis and in-vivo evaluation of [125I]IBT: A single-photon emission computed tomography radiotracer for alpha7-nicotinic acetylcholine receptor imaging. Nucl. Med. Commun. 2017, 38, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Fang, Y.; Wang, H.; Gao, H.; Jiang, G.; Liu, J.; Xue, Q.; Qi, Y.; Cao, M.; Qiang, B.; et al. Design, synthesis and biological evaluation of 1,4-Diazobicylco[3.2.2]nonane derivatives as alpha7-Nicotinic acetylcholine receptor PET/CT imaging agents and agonists for Alzheimer’s disease. Eur. J. Med. Chem. 2018, 159, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Teodoro, R.; Scheunemann, M.; Wenzel, B.; Peters, D.; Deuther-Conrad, W.; Brust, P. Synthesis and radiofluorination of novel fluoren-9-one based derivatives for the imaging of alpha7 nicotinic acetylcholine receptor with PET. Bioorg. Med. Chem. Lett. 2018, 28, 1471–1475. [Google Scholar] [CrossRef]
- Sarasamkan, J.; Scheunemann, M.; Apaijai, N.; Palee, S.; Parichatikanond, W.; Arunrungvichian, K.; Fischer, S.; Chattipakorn, S.; Deuther-Conrad, W.; Schuurmann, G.; et al. Varying Chirality Across Nicotinic Acetylcholine Receptor Subtypes: Selective Binding of Quinuclidine Triazole Compounds. Acs Med. Chem. Lett. 2016, 7, 890–895. [Google Scholar] [CrossRef] [Green Version]
- Schrimpf, M.R.; Sippy, K.B.; Briggs, C.A.; Anderson, D.J.; Li, T.; Ji, J.; Frost, J.M.; Surowy, C.S.; Bunnelle, W.H.; Gopalakrishnan, M.; et al. SAR of alpha7 nicotinic receptor agonists derived from tilorone: Exploration of a novel nicotinic pharmacophore. Bioorg. Med. Chem. Lett. 2012, 22, 1633–1638. [Google Scholar] [CrossRef]
- Horti, A.G.; Gao, Y.; Kuwabara, H.; Wang, Y.; Abazyan, S.; Yasuda, R.P.; Tran, T.; Xiao, Y.; Sahibzada, N.; Holt, D.P.; et al. 18F-ASEM, a radiolabeled antagonist for imaging the alpha7-nicotinic acetylcholine receptor with PET. J. Nucl. Med. 2014, 55, 672–677. [Google Scholar] [CrossRef] [Green Version]
- Teodoro, R.; Scheunemann, M.; Deuther-Conrad, W.; Wenzel, B.; Fasoli, F.M.; Gotti, C.; Kranz, M.; Donat, C.K.; Patt, M.; Hillmer, A.; et al. A Promising PET Tracer for Imaging of alpha(7) Nicotinic Acetylcholine Receptors in the Brain: Design, Synthesis, and In Vivo Evaluation of a Dibenzothiophene-Based Radioligand. Molecules 2015, 20, 18387–18421. [Google Scholar] [CrossRef] [Green Version]
- Horti, A.G. Development of [(18)F]ASEM, a specific radiotracer for quantification of the alpha7-nAChR with positron-emission tomography. Biochem. Pharmacol. 2015, 97, 566–575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Y.; Mease, R.C.; Olson, T.T.; Kellar, K.J.; Dannals, R.F.; Pomper, M.G.; Horti, A.G. [(125)I]Iodo-ASEM, a specific in vivo radioligand for alpha7-nAChR. Nucl. Med. Biol. 2015, 42, 488–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, D.F.; Kuwabara, H.; Pomper, M.; Holt, D.P.; Brasic, J.R.; George, N.; Frolov, B.; Willis, W.; Gao, Y.; Valentine, H.; et al. Human brain imaging of alpha7 nAChR with [(18)F]ASEM: A new PET radiotracer for neuropsychiatry and determination of drug occupancy. WinnieBbb13 2014, 16, 730–738. [Google Scholar]
- Hillmer, A.T.; Li, S.; Zheng, M.Q.; Scheunemann, M.; Lin, S.F.; Nabulsi, N.; Holden, D.; Pracitto, R.; Labaree, D.; Ropchan, J.; et al. PET imaging of alpha7 nicotinic acetylcholine receptors: A comparative study of [18F]ASEM and [18F]DBT-10 in nonhuman primates, and further evaluation of [18F]ASEM in humans. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1042–1050. [Google Scholar] [CrossRef]
- Hillmer, A.T.; Zheng, M.Q.; Li, S.; Scheunemann, M.; Lin, S.F.; Holden, D.; Labaree, D.; Ropchan, J.; Teodoro, R.; Deuther-Conrad, W.; et al. PET imaging evaluation of [(18)F]DBT-10, a novel radioligand specific to alpha7 nicotinic acetylcholine receptors, in nonhuman primates. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 537–547. [Google Scholar] [CrossRef] [Green Version]
- Wong, D.F.; Kuwabara, H.; Horti, A.G.; Roberts, J.M.; Nandi, A.; Cascella, N.; Brasic, J.; Weerts, E.M.; Kitzmiller, K.; Phan, J.A.; et al. Brain PET Imaging of alpha7-nAChR with [18F]ASEM: Reproducibility, Occupancy, Receptor Density, and Changes in Schizophrenia. Int. J. Neuropsychopharmacol. /Off. Sci. J. Coll. Int. Neuropsychopharmacol. 2018, 21, 656–667. [Google Scholar]
- Coughlin, J.M.; Du, Y.; Rosenthal, H.B.; Slania, S.; Min Koo, S.; Park, A.; Solomon, G.; Vranesic, M.; Antonsdottir, I.; Speck, C.L.; et al. The distribution of the alpha7 nicotinic acetylcholine receptor in healthy aging: An in vivo positron emission tomography study with [(18)F]ASEM. NeuroImage 2017, 165, 118–124. [Google Scholar] [CrossRef]
- Coughlin, J.; Du, Y.; Crawford, J.L.; Rubin, L.H.; Behnam Azad, B.; Lesniak, W.G.; Horti, A.G.; Schretlen, D.J.; Sawa, A.; Pomper, M.G. The availability of the alpha7 nicotinic acetylcholine receptor in recent-onset psychosis: A study using (18)F-ASEM PET. J. Nucl. Med. 2018. [Google Scholar] [CrossRef] [Green Version]
- Coughlin, J.M.; Rubin, L.H.; Du, Y.; Rowe, S.P.; Crawford, J.L.; Rosenthal, H.B.; Frey, S.M.; Marshall, E.S.; Shinehouse, L.K.; Chen, A.; et al. High availability of the alpha7 nicotinic acetylcholine receptor in brains of individuals with mild cognitive impairment: A pilot study using (18)F-ASEM PET. J. Nucl. Med. 2019. [Google Scholar] [CrossRef]
- Vetel, S.; Vercouillie, J.; Buron, F.; Vergote, J.; Tauber, C.; Busson, J.; Chicheri, G.; Routier, S.; Serriere, S.; Chalon, S. Longitudinal PET Imaging of alpha7 Nicotinic Acetylcholine Receptors with [(18)F]ASEM in a Rat Model of Parkinson’s Disease. WinnieBbb13 2019. [Google Scholar] [CrossRef]
- Wu, J.; Liu, Q.; Tang, P.; Mikkelsen, J.D.; Shen, J.; Whiteaker, P.; Yakel, J.L. Heteromeric alpha7beta2 Nicotinic Acetylcholine Receptors in the Brain. Trends Pharm. Sci. 2016, 37, 562–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomsen, M.S.; Zwart, R.; Ursu, D.; Jensen, M.M.; Pinborg, L.H.; Gilmour, G.; Wu, J.; Sher, E.; Mikkelsen, J.D. alpha7 and beta2 Nicotinic Acetylcholine Receptor Subunits Form Heteromeric Receptor Complexes that Are Expressed in the Human Cortex and Display Distinct Pharmacological Properties. PLoS ONE 2015, 10, e0130572. [Google Scholar] [CrossRef] [PubMed]
- Moretti, M.; Zoli, M.; George, A.A.; Lukas, R.J.; Pistillo, F.; Maskos, U.; Whiteaker, P.; Gotti, C. The novel alpha7beta2-nicotinic acetylcholine receptor subtype is expressed in mouse and human basal forebrain: Biochemical and pharmacological characterization. Mol. Pharmacol. 2014, 86, 306–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zwart, R.; Strotton, M.; Ching, J.; Astles, P.C.; Sher, E. Unique pharmacology of heteromeric alpha7beta2 nicotinic acetylcholine receptors expressed in Xenopus laevis oocytes. Eur. J. Pharmacol. 2014, 726C, 77–86. [Google Scholar] [CrossRef]
- Patel, S.; Hamill, T.G.; Connolly, B.; Jagoda, E.; Li, W.; Gibson, R.E. Species differences in mGluR5 binding sites in mammalian central nervous system determined using in vitro binding with [18F]F-PEB. Pet§Cholin2 2007, 34, 1009–1017. [Google Scholar] [CrossRef]
- Fujita, M.; Imaizumi, M.; Zoghbi, S.S.; Fujimura, Y.; Farris, A.G.; Suhara, T.; Hong, J.; Pike, V.W.; Innis, R.B. Kinetic analysis in healthy humans of a novel positron emission tomography radioligand to image the peripheral benzodiazepine receptor, a potential biomarker for inflammation. NeuroImage 2008, 40, 43–52. [Google Scholar] [CrossRef] [Green Version]
- Van de Bittner, G.C.; Ricq, E.L.; Hooker, J.M. A philosophy for CNS radiotracer design. Acc Chem. Res. 2014, 47, 3127–3134. [Google Scholar] [CrossRef] [Green Version]
- Laruelle, M.; Slifstein, M.; Huang, Y. Relationships between radiotracer properties and image quality in molecular imaging of the brain with positron emission tomography. Nicotin4 2003, 5, 363–375. [Google Scholar] [CrossRef]
- Magnussen, J.H.; Ettrup, A.; Donat, C.K.; Peters, D.; Pedersen, M.H.F.; Knudsen, G.M.; Mikkelsen, J.D. Radiosynthesis and in vitro validation of 3H-NS14492 as a novel high affinity α7 nicotinic acetylcholine receptor radioligand Mol. Cell. Neurosci. 2014. [Google Scholar] [CrossRef]
- Anderson, D.J.; Bunnelle, W.; Surber, B.; Du, J.; Surowy, C.; Tribollet, E.; Marguerat, A.; Bertrand, D.; Gopalakrishnan, M. [3H]A-585539 [(1S,4S)-2,2-dimethyl-5-(6-phenylpyridazin-3-yl)-5-aza-2-azoniabicyclo[2.2.1]hept ane], a novel high-affinity alpha7 neuronal nicotinic receptor agonist: Radioligand binding characterization to rat and human brain. J. Pharmacol. Exp. Ther. 2008, 324, 179–187. [Google Scholar] [CrossRef]
- Magnussen, J.H.; Ettrup, A.; Donat, C.K.; Peters, D.; Pedersen, M.H.; Knudsen, G.M.; Mikkelsen, J.D. Radiosynthesis and in vitro validation of (3)H-NS14492 as a novel high affinity alpha7 nicotinic receptor radioligand. Eur. J. Pharmacol. 2015, 762, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Bitner, R.S.; Bunnelle, W.H.; Anderson, D.J.; Briggs, C.A.; Buccafusco, J.; Curzon, P.; Decker, M.W.; Frost, J.M.; Gronlien, J.H.; Gubbins, E.; et al. Broad-spectrum efficacy across cognitive domains by alpha7 nicotinic acetylcholine receptor agonism correlates with activation of ERK1/2 and CREB phosphorylation pathways. J. Neurosci. 2007, 27, 10578–10587. [Google Scholar] [CrossRef] [PubMed]
- Biton, B.; Bergis, O.E.; Galli, F.; Nedelec, A.; Lochead, A.W.; Jegham, S.; Godet, D.; Lanneau, C.; Santamaria, R.; Chesney, F.; et al. SSR180711, a novel selective alpha7 nicotinic receptor partial agonist: (1) binding and functional profile. Neuropsychopharmacology 2007, 32, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hauser, T.A.; Kucinski, A.; Jordan, K.G.; Gatto, G.J.; Wersinger, S.R.; Hesse, R.A.; Stachowiak, E.K.; Stachowiak, M.K.; Papke, R.L.; Lippiello, P.M.; et al. TC-5619: An alpha7 neuronal nicotinic receptor-selective agonist that demonstrates efficacy in animal models of the positive and negative symptoms and cognitive dysfunction of schizophrenia. Biochem. Pharmacol. 2009, 78, 803–812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prickaerts, J.; van Goethem, N.P.; Chesworth, R.; Shapiro, G.; Boess, F.G.; Methfessel, C.; Reneerkens, O.A.; Flood, D.G.; Hilt, D.; Gawryl, M.; et al. EVP-6124, a novel and selective alpha7 nicotinic acetylcholine receptor partial agonist, improves memory performance by potentiating the acetylcholine response of alpha7 nicotinic acetylcholine receptors. Neuropharmacology 2012, 62, 1099–1110. [Google Scholar] [CrossRef] [PubMed]
- Meyer, E.M.; Tay, E.T.; Papke, R.L.; Meyers, C.; Huang, G.L.; de Fiebre, C.M. 3-[2,4-Dimethoxybenzylidene]anabaseine (DMXB) selectively activates rat alpha7 receptors and improves memory-related behaviors in a mecamylamine-sensitive manner. Brain Res. 1997, 768, 49–56. [Google Scholar] [CrossRef]
- Nielsen, B.E.; Minguez, T.; Bermudez, I.; Bouzat, C. Molecular function of the novel α7β2 nicotinic receptor. Cell. Mol. Life Sci. Cmls 2018, 75, 2457–2471. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Huang, Y.; Shen, J.; Steffensen, S.; Wu, J. Functional alpha7beta2 nicotinic acetylcholine receptors expressed in hippocampal interneurons exhibit high sensitivity to pathological level of amyloid beta peptides. Bmc Neurosci. 2012, 13, 155. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Huang, Y.; Xue, F.; Simard, A.; DeChon, J.; Li, G.; Zhang, J.; Lucero, L.; Wang, M.; Sierks, M.; et al. A novel nicotinic acetylcholine receptor subtype in basal forebrain cholinergic neurons with high sensitivity to amyloid peptides. J. Neurosci. 2009, 29, 918–929. [Google Scholar] [CrossRef]
- Khiroug, S.S.; Harkness, P.C.; Lamb, P.W.; Sudweeks, S.N.; Khiroug, L.; Millar, N.S.; Yakel, J.L. Rat nicotinic ACh receptor alpha7 and beta2 subunits co-assemble to form functional heteromeric nicotinic receptor channels. J Physiol 2002, 540, 425–434. [Google Scholar] [CrossRef]
- Murray, T.A.; Bertrand, D.; Papke, R.L.; George, A.A.; Pantoja, R.; Srinivasan, R.; Liu, Q.; Wu, J.; Whiteaker, P.; Lester, H.A.; et al. alpha7beta2 nicotinic acetylcholine receptors assemble, function, and are activated primarily via their alpha7-alpha7 interfaces. Mol. Pharmacol. 2012, 81, 175–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mowrey, D.D.; Liu, Q.; Bondarenko, V.; Chen, Q.; Seyoum, E.; Xu, Y.; Wu, J.; Tang, P. Insights into distinct modulation of α7 and α7β2 nicotinic acetylcholine receptors by the volatile anesthetic isoflurane. J. Biol. Chem. 2013, 288, 35793–35800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teodoro, R.; Moldovan, R.P.; Lueg, C.; Gunther, R.; Donat, C.K.; Ludwig, F.A.; Fischer, S.; Deuther-Conrad, W.; Wunsch, B.; Brust, P. Radiofluorination and biological evaluation of N-aryl-oxadiazolyl-propionamides as potential radioligands for PET imaging of cannabinoid CB2 receptors. Org. Med. Chem. Lett. 2013, 3, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Innis, R.B.; Cunningham, V.J.; Delforge, J.; Fujita, M.; Gjedde, A.; Gunn, R.N.; Holden, J.; Houle, S.; Huang, S.-C.; Ichise, M.; et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J. Cereb. Blood Flow Metab: Off. J. Int. Soc. Cereb. Blood Flow Metab. 2007, 27, 1533–1539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallezot, J.-D.; Weinzimmer, D.; Nabulsi, N.; Lin, S.-F.; Fowles, K.; Sandiego, C.; McCarthy, T.J.; Maguire, R.P.; Carson, R.E.; Ding, Y.-S. Evaluation of [(11)C]MRB for assessment of occupancy of norepinephrine transporters: Studies with atomoxetine in non-human primates. NeuroImage 2011, 56, 268–279. [Google Scholar] [CrossRef] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Ward, J.M.; Cockcroft, V.B.; Lunt, G.G.; Smillie, F.S.; Wonnacott, S. Methyllycaconitine: A selective probe for neuronal alpha-bungarotoxin binding sites. Febs Lett. 1990, 270, 45–48. [Google Scholar] [CrossRef] [Green Version]
- Gillings, N. A restricted access material for rapid analysis of [(11)C]-labeled radiopharmaceuticals and their metabolites in plasma. Nucl. Med. Biol. 2009, 36, 961–965. [Google Scholar] [CrossRef]
- Kornum, B.R.; Lind, N.M.; Gillings, N.; Marner, L.; Andersen, F.; Knudsen, G.M. Evaluation of the novel 5-HT4 receptor PET ligand [11C]SB207145 in the Gottingen minipig. J. Cereb. Blood Flow Metab. 2009, 29, 186–196. [Google Scholar] [CrossRef] [Green Version]
- Villadsen, J.; Hansen, H.D.; Jorgensen, L.M.; Keller, S.H.; Andersen, F.L.; Petersen, I.N.; Knudsen, G.M.; Svarer, C. Automatic delineation of brain regions on MRI and PET images from the pig. J. Neurosci. Methods 2018, 294, 51–58. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available. |




| Tracer | Structure |
|---|---|
| [11C]CHIBA-1001 |  |
| [11C]A-582941 |  |
| [18F]NS14490 |  |
| [11C]NS14492 |  |
| [18F]ASEM |  |
| [18F]DBT-10 |  |
| [125I]ASEM |  |
| Ligand (10 µMol/L) | [125I]Iodo-ASEM Binding in the Pig Cortex, Layers 1–3 (%, mean ± S.E.M.) | [125I]Iodo-ASEM Binding in the Pig Cortex, Layers 4–6 (%, mean ± S.E.M.) |
|---|---|---|
| NS14492 | 4.04 ± 0.55 | 9.25 ± 0.83 |
| TC-5619 | 7.88 ± 1.65 | 7.60 ± 0.30 |
| EVP-6124 | 2.50 ± 0.20 | 5.50 ± 0.07 |
| A-582941 | 3.22 ± 0.28 | 4.44 ± 0.93 |
| SSR-180,711 | 2.92 ± 0.35 | 3.53 ± 0.50 |
| GTS-21 | 30.92 ± 2.55 | 31.39 ± 2.15 |
| MLA | 20.46 ± 2.18 | 18.16 ± 2.41 |
| Comparison of Baseline VT Values. | ||||
|---|---|---|---|---|
| Kinetic Modelling | Animal 1 | Animal 2 | ||
| 0–90 min | VT | VT/fP | VT | VT/fP |
| Frontal cortex | 7.87 | 43.70 | 3.75 | 41.66 |
| Somatosensory cortex | 8.33 | 46.27 | 4.15 | 46.14 |
| Occipital cortex | 8.03 | 44.63 | 3.77 | 41.86 |
| Remaining cortex | 7.63 | 42.37 | 3.78 | 41.94 |
| Thalamus | 8.83 | 49.06 | 4.12 | 45.73 |
| Striatum | 7.41 | 41.17 | 3.77 | 41.94 |
| Hippocampus | 7.53 | 41.83 | 3.59 | 39.93 |
| Cerebellum | 6.66 | 36.99 | 3.16 | 35.07 |
| Comparison of VT Values at Baseline and After Pre-treatment with SSR-180,711 | ||||
| Kinetic Modelling | Animal 1 | Animal 3 | ||
| 0–150 min | VT | VT/fP | VT | VT/fP |
| Frontal cortex | 6.73 | 37.38 | 3.61 | 22.57 |
| Somatosensory cortex | 7.26 | 40.35 | 3.94 | 24.60 |
| Occipital cortex | 6.96 | 38.65 | 3.90 | 24.40 |
| Remaining cortex | 6.68 | 37.08 | 3.60 | 22.50 |
| Thalamus | 7.42 | 41.24 | 4.34 | 27.15 |
| Striatum | 6.51 | 36.19 | 3.83 | 23.92 |
| Hippocampus | 6.55 | 36.41 | 3.37 | 21.09 |
| Cerebellum | 5.55 | 30.82 | 3.39 | 21.17 |
| Details | Animal 1 | Animal 2 | Animal 3 |
|---|---|---|---|
| Type of experiment | Baseline | Baseline | SSR-180,711; 1 mg/kg |
| Scan length | 240 min | 90 min | 150 min |
| Molar activity | 20 GBq/µmol | 345 GBq/µmol | 388 GBq/µmol |
| Injected activity | 99 MBq | 335 MBq | 189 MBq |
| Injected mass | 1.78 μg | 0.35 μg | 0.18 μg |
| Free plasma fraction | 18% | 16% | 9% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donat, C.K.; Hansen, H.H.; Hansen, H.D.; Mease, R.C.; Horti, A.G.; Pomper, M.G.; L’Estrade, E.T.; Herth, M.M.; Peters, D.; Knudsen, G.M.; et al. In Vitro and In Vivo Characterization of Dibenzothiophene Derivatives [125I]Iodo-ASEM and [18F]ASEM as Radiotracers of Homo- and Heteromeric α7 Nicotinic Acetylcholine Receptors. Molecules 2020, 25, 1425. https://doi.org/10.3390/molecules25061425
Donat CK, Hansen HH, Hansen HD, Mease RC, Horti AG, Pomper MG, L’Estrade ET, Herth MM, Peters D, Knudsen GM, et al. In Vitro and In Vivo Characterization of Dibenzothiophene Derivatives [125I]Iodo-ASEM and [18F]ASEM as Radiotracers of Homo- and Heteromeric α7 Nicotinic Acetylcholine Receptors. Molecules. 2020; 25(6):1425. https://doi.org/10.3390/molecules25061425
Chicago/Turabian StyleDonat, Cornelius K., Henrik H. Hansen, Hanne D. Hansen, Ronnie C. Mease, Andrew G. Horti, Martin G. Pomper, Elina T. L’Estrade, Matthias M. Herth, Dan Peters, Gitte M. Knudsen, and et al. 2020. "In Vitro and In Vivo Characterization of Dibenzothiophene Derivatives [125I]Iodo-ASEM and [18F]ASEM as Radiotracers of Homo- and Heteromeric α7 Nicotinic Acetylcholine Receptors" Molecules 25, no. 6: 1425. https://doi.org/10.3390/molecules25061425
APA StyleDonat, C. K., Hansen, H. H., Hansen, H. D., Mease, R. C., Horti, A. G., Pomper, M. G., L’Estrade, E. T., Herth, M. M., Peters, D., Knudsen, G. M., & Mikkelsen, J. D. (2020). In Vitro and In Vivo Characterization of Dibenzothiophene Derivatives [125I]Iodo-ASEM and [18F]ASEM as Radiotracers of Homo- and Heteromeric α7 Nicotinic Acetylcholine Receptors. Molecules, 25(6), 1425. https://doi.org/10.3390/molecules25061425