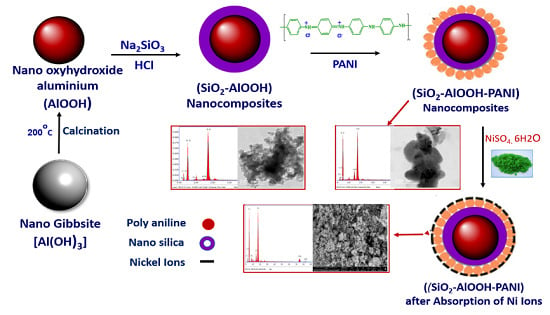
Synthesis and Characterization of Silica-Coated Oxyhydroxide Aluminum/Doped Polymer Nanocomposites: A Comparative Study and Its Application as a Sorbent
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural and Surface Characterization of the Adsorbent
2.1.1. FT-IR Study
2.1.2. X-Ray Diffraction Study
2.1.3. SEM-EDX Study
2.1.4. Transition Electron Microscopy Study
2.2. Effect of pH
2.3. Effect of Contact Time
2.4. Effect of Ni(II) Ion Concentration
2.5. Kinetics Models
2.5.1. Pseudo First-Order Reaction Kinetics
2.5.2. Pseudo Second-Order Reaction
2.5.3. Mories–Weber Kinetic Equation
2.6. Isotherm Model
Dubinin–Radusekevisch–Kanager Isotherm
2.7. Sorption Thermodynamics
2.8. Cost Estimation per kg of the Adsorbent
3. Experimental Procedures
3.1. Materials
3.2. Synthesis of Nano Oxyhydroxide Aluminum
3.3. Synthesis of Nano-Silica-Coated Oxyhydroxide Aluminum Nanocomposite
3.4. Synthesis of Polyaniline
3.5. Synthesis of Nano-Silica-Coated Oxyhydroxide Aluminum Doped Polyaniline Nanocomposite
3.6. Surface Characterization of the Nanocomposites
3.6.1. Instruments
3.6.2. Adsorption Studies of Nickel Ions
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cheraghi, M.; Lorestani, B.; Yousefi, N. Effect of waste water on heavy metal accumulation in Hamedan Province vegetables. Int. J. Bot. 2009, 5, 190–193. [Google Scholar] [CrossRef] [Green Version]
- Islam, A.; Awual, R.; Angove, M.J. A Review on Nickel (II) Adsorption in Single and Binary Component Systems and Future Path; Science Publishing: New York, NY, USA, 2019; pp. 1–64. [Google Scholar]
- Vinni, N.T.; Vinoth, K.V.; Senthil, K.P.; Christy, C.; Sailavanyaa, S.; Dhanya, V.; Saravanan, A.; Vasanth, K.V.; Sivanesan, S. Review on nano adsorbents: A solution for heavy metal removal from wastewater. IET Nanobiotechnol. 2016, 13, 2025–2036. [Google Scholar]
- Fuqiang, A.; Baojiao, G. Studies an chelating adsorption properties of novel composite material polyethylene imine/silica gel for heavy ions. Appl. Surf. Sci. 2006, 253, 1946–1952. [Google Scholar]
- Qdais, H.A.; Moussa, H. Removal of heavy metals from wastewater by membrane processes: A comparative study. Desalination 2004, 164, 105–110. [Google Scholar] [CrossRef]
- Arabulut, K.; Karabakan, S.A.; Denizli, A.; Yurum, Y. Batch Removal of Copper(II) and Zinc(II) from Aqueous Solutions with Low-Rank Turkish Coals. Sep. Purif. Technol. 2000, 18, 177–184. [Google Scholar] [CrossRef]
- Srisuwan, G.; Thongchai, P. Removal of heavy metals from electroplating wastewater by membrane. Membr. Sci. Technol. 2002, 24, 965–976. [Google Scholar]
- Rao, K.D.; Sarangi, D.P.; Chaudhury, G. Treatment of wastewater containing Cu, Zn, Ni and Co ions using Duolite. J. Chem. Technol. Biotechnol. 2002, 77, 1107–1113. [Google Scholar]
- Hussein, H. Experimental Studies of the Electrochemical Deposition of Copper; LAP LAMBERT Academic Publishing: Saarbrücken, Germany, 2019. [Google Scholar]
- Pehlivan, E.; Cetin, S. Sorption of Cr (VI) ions on two Lewatit-anion exchange resins and their quantitative determination using UV–visible spectrophotometer. J. Hazard. Mater. 2009, 163, 448–453. [Google Scholar] [CrossRef]
- FengTing, L.I.; Xia, L.I.; Zhang, B.; Ouyang, Q. Removal of heavy metals in effluent by adsorption and coagulation. Chin. Chem. Lett. 2004, 15, 83–86. [Google Scholar]
- Qingrui, Z.; Shuaiqi, Z.; Zhixue, Z.; Meng, L.; Xiaofeng, Y.; Yanping, Z.; Yun, W.; Qiuming, P. Highly effective lead (II) removal by sustainable alkaline activated β-lactoglobulin nanofibrils from whey protein. J. Clean. Prod. 2020, 255, 120297. [Google Scholar] [CrossRef]
- Qingrui, Z.; Sreenath, B.; Yiping, C.; Stephan, H.; Joze, F.A.; Qiuming, P.; Raffaele, M. Selective and Efficient Removal of Fluoride from Water: In Situ Engineered Amyloid Fibril/ZrO2 Hybrid Membranes. Angew. Chem. 2019, 58, 6012–6016. [Google Scholar]
- Hui, Q.; Wenxiang, N.; Haihan, Z.; Kai, C.; Jiacheng, Y. Fabrication and evaluation of a regenerable HFO-doped agricultural waste for enhanced adsorption affinity towards phosphate. Sci. Total Environ. 2020, 703, 135493. [Google Scholar] [CrossRef]
- Qingrui, Z.; Qinggang, Y.; Pikky, P.; Yixuan, L.; Zikang, W.; Tifeng, J.; Qiuming, P. Highly Efficient Lead (II) Sequestration Using the Size-controllable Polydopamine Microspheres with Superior Application Capability and Rapid Capture. ACS Sustain. Chem. Eng. 2017, 5, 4161–4170. [Google Scholar]
- Sharma, Y.C.; Srivastava, V.; Mukherjee, A.K. Synthesis and application of nano-Al2O3 powder for the reclamation of hexavalent chromium from aqueous solutions. J. Chem. Eng. Data 2010, 55, 2390. [Google Scholar] [CrossRef]
- Abiyu, K.; Adane, D.; Ramesh, D. Removal of Methyl Violet from Synthetic Wastewater Using Nano Aluminium Oxyhydroxide. IJERT 2016, 12, 22–28. [Google Scholar]
- Farhad, S.; Keivan, T.; Changiz, K.; Alireza, J. Preparation of Modified nano-SiO2 by Bismuth and Iron as a novel Remover of Methylene Blue from Water Solution. J. Mex. Chem. Soc. 2017, 61, 250–259. [Google Scholar]
- George, Z.K.; Eleni, A.D. Mercury(II) Removal with Modified Magnetic Chitosan Adsorbents. Molecules 2013, 18, 6193–6214. [Google Scholar]
- Zou, H.; Wu, S.S.; Shen, J. Polymer/silica nanocomposites: Preparation, characterization, properties, and applications. Chem. Rev. 2008, 108, 3893–3957. [Google Scholar] [CrossRef]
- Roosz, N.; Euvard, M.; Lakard, B.; Buron, C.C.; Martin, N.; Viau, L. Synthesis and characterization of polyaniline-silica, composites: Raspberry vs core-shell structures. Where do we stand? J. Colloid Interface Sci. 2017, 502, 184–192. [Google Scholar] [CrossRef]
- Rathinam, K.; Meenakshi, S. Removal of hexavalent chromium ions using polyaniline/silica gel composite. J. Water Process. Eng. 2014, 1, 37–45. [Google Scholar]
- Inas, A.; Najlaa, S.; Hussein, H.; Ahmed, R. Environmentally Friendly Mesoporous Nanocomposite Prepared from Al-Dross Waste with Remarkable Adsorption Ability for Toxic Anionic Dye. J. Chem. 2019, 121, 1126. [Google Scholar]
- Leonard, K.C.; Genthe, J.R.; Sanippo, J.L.; Zeltner, W.A.; Anderson, M.A. Synthesis and characterization of asymmetric electrochemical capacitive deionization materials using nanoporous silicon dioxide and magnesium doped aluminum oxide. Electrochim. Acta 2009, 54, 5286–5291. [Google Scholar] [CrossRef]
- Peige, Q.; Yixin, Y.; Xiaoting, Z.; Jiahua, N.; Hui, Y.; Shufang, T.; Jinhua, Z.; Minghua, L. Highly Efficient, Rapid, and Simultaneous Removal of Cationic Dyes from Aqueous Solution Using Monodispersed Mesoporous Silica Nanoparticles as the Adsorbent. Nanomaterials 2018, 8, 4. [Google Scholar]
- Chiharu, T.; Shinya, S.; Daisuke, H.; Sayaka, I. Silicate Removal in Aluminum Hydroxide Co Precipitation. Process. Mater. 2014, 7, 1084–1096. [Google Scholar]
- Shiba, K.; Sugiyama, T.; Takei, T.; Yoshikawa, G. Controlled growth of silica–titania hybrid functional nanoparticles through a multistep microfluidic approach. Chem. Commun. 2015, 51, 15854. [Google Scholar] [CrossRef]
- Feng, X.; Yang, G.; Liu, Y.; Hou, W.; Zhu, J. Synthesis of polyaniline/MCM-41 composite through surface polymerization of aniline. Appl. Polym. Sci. 2006, 101, 2088. [Google Scholar] [CrossRef]
- Vijayalekshmi, V.; Dipak, K. Hybrid composite membranes of chitosan/sulfonated polyaniline/silica as polymer electrolyte membrane for fuel cells. Carbohydr. Polym. 2018, 179, 152–163. [Google Scholar]
- Noh, J.; Yoon, C.M.; Jang, J. Enhanced electrorheological activity of polyaniline coated mesoporous silica with high aspect ratio. Colloid Interface Sci. 2016, 470, 237. [Google Scholar] [CrossRef]
- El-Didamony, H.; El-Sokkary, T.M.; Khalil, K.A.; Heikal, M.; Ahmed, I.A. Hydration mechanisms of calcium sulphoaluminate C4A3, C4A phase and active belite β-C2S. Ceram. Silikáty 2012, 56, 389–395. [Google Scholar]
- Dmitry, V.; Vyacheslav, P.; Alexandra, M.; Margarita, G.; Mark, Z.; Irina, D.; Yuri, L.; Valerii, B.; Konstantin, C. Extraction of Aluminium by Autoclave Hydrochloric Acid Leaching of Oxyhydroxide aluminum-Kaolinite Bauxite Conference, Proceedings of the TMS 145th Annual Meeting & Exhibition: Nashville, TN, USA, 14–18 February 2016; Springer: Berlin, Germany, 2016; pp. 23–28. [Google Scholar]
- Kondawar, S.B.; Deshpande, M.D.; Agrawal, S.P. Transport Properties of Conductive Polyaniline Nanocomposites Based on Carbon Nanotubes. Int. J. Compos. Mater. 2012, 2, 32–36. [Google Scholar] [CrossRef] [Green Version]
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H.M. Dye and its removal from aqueous solution by adsorption: A review. Adv. Colloid Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef]
- Filip, C.; Przemysław, B.; Karolina, W.; Katarzyna, S.; Magdalena, N.; Teofil, J. Adsorption of Ni (II) from model solutions using co-precipitated inorganic oxides. Adsorption 2013, 19, 423–434. [Google Scholar]
- Gérrard, E.; Sridevi, B.; Suraj Kumar, T.; Mrutyunjay, S.; Derek, F. Kinetic and Adsorption Behaviour of Aqueous Cadmium Using a 30 nm Hydroxyapatite Based Powder Synthesized Via a Combined Ultrasound and Microwave Based Technique. Phys. Chem. 2016, 6, 11–22. [Google Scholar]
- Mobasherpour, I.; Salahi, E.; Pazouki, M. Removal of divalent cadmium cations by means of synthetic nano-crystallite hydroxyapatite. Desalination 2011, 266, 142–148. [Google Scholar] [CrossRef]
- Abdel-Moneum, M.; Ahmeda, E.; Alib, G. Adsorption Separation of Nickel from Wastewater by using Olive Stones. Adv. J. Chem. Sect. A 2019, 2, 79–93. [Google Scholar]
- Bilge, A.; Sevil, V. Kinetics and equilibrium studies for the removal of nickel and zinc from aqueous solutions by ion exchange resins. J. Hazard. Mater. 2009, 167, 482–488. [Google Scholar]
- Millar, G.J.; Couperthwaite, S.J.; Dawes, L.A.; Thompson, S.; Spencer, J. Activated alumina for the removal of fuoride ions from high alkalinity groundwater: New insights from equilibrium and column studies with multicomponent solutions. Sep. Purif. Technol. 2017, 187, 14–24. [Google Scholar] [CrossRef] [Green Version]
- Lagergren, S. Zurtheorie der sogenannten adsorption gel sterstoffe. K. Sven. Vetensk. Handl. 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process. Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Aljeboree, M.; Alshirifi, N.; Alkaim, F. Kinetics and equilibrium study for the adsorption of textile dyes on coconut shell activated carbon. Arab. J. Chem. 2017, 10, 3381. [Google Scholar] [CrossRef] [Green Version]
- Elwakeel, K.Z.; El-Bindary, A.A.; Koutac, E.Y.; Gui, E. Functionalization of polyacrylonitrile/Na-Y-zeolite composite with amidoxime groups for the sorption of Cu(II), Cd(II) and Pb(II) metal ions. Chem. Eng. 2018, 332, 727–736. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. The Kinetics of Sorption of Divalent Metal Ions onto Sphagnum Moss Peat. Water Res. 2000, 34, 735–742. [Google Scholar] [CrossRef]
- Meenakshi, S.; Viswanathan, N. Identification of selective ion exchange resin for fluoride sorption. J. Colloids Interface Sci. 2007, 308, 438–450. [Google Scholar] [CrossRef]
- Israa, I.N.; Hilal, W.S. Adsorption of Eriochrom Black T Azo Dye onto Nanosized Anatase TiO2. J. Environ. Eng. Sci. 2015, 2, 86–92. [Google Scholar]
- Ho, Y.S. Effect of pH on lead removal from water using tree fern as the sorbent. Bioresour. Technol. 2005, 96, 1292–1296. [Google Scholar] [CrossRef] [PubMed]
- Veliev, E.V.; Öztürk, T.; Veli, S.; Fatullayev, A.G. Application of diffusion model for adsorption of azo reactrive dye on pumice. Pol. J. Environ. Stud. 2006, 15, 347–353. [Google Scholar]
- Daneshvar, N.; Salari, D.; Aber, S. Chromium adsorption and Cr (VI) reduction to trivalent chromium in aqueous solutions by soya cake. J. Hazard. Mater. 2002, 94, 49–61. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Uber die adsorption in losungen. Z. Phys. Chem. 1906, 57, 385–470. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Özcan, A.; Öncü, E.M.; Özcan, A.S. Kinetics, isotherm and thermodynamic studies of adsorption of Acid Blue 193 from aqueous solutions onto natural sepiolite. Colloids Surf. A 2006, 277, 90–97. [Google Scholar] [CrossRef]
- Elgarahy, A.M.; Elwakeel, K.Z.; Elshoubaky, G.A.; Mohammad, S.H. Microwave-accelerated sorption of cationic dyes onto green marine algal biomass. Environ. Sci. Pollut. Res. 2019, 26, 22704–22722. [Google Scholar] [CrossRef] [PubMed]
- Salvestrini, S.; Leone, V.; Iovino, P.; Canzano, S.; Capasso, S. Considerations about the correct evaluation of sorption thermodynamic parameters from equilibrium isotherms. J. Chem. Thermodyn. 2014, 68, 310–316. [Google Scholar] [CrossRef]
- Wentong, Z.; Jing, Z.; Wei, W.; Lirong, M.; Jianjun, Z.; Jimin, X. Comparative study of modified/non-modified aluminum and silica aerogels for anionic dye adsorption performance. RSC Adv. 2018, 8, 29129. [Google Scholar]
- Munagapati, V.S.; Yarramuthi, V.; Kim, Y.; Lee, K.M.; SuKim, D. Removal of anionic dyes (Reactive Black 5 and Congo Red) from aqueous solutions using banana peel powder as an adsorbent. Ecotoxicol. Environ. Saf. 2018, 148, 601–607. [Google Scholar] [CrossRef] [PubMed]
- US Research Nanomaterials, Inc. Available online: https://www.us-nano.com/inc/sdetail/149 (accessed on 18 February 2020).
- Peters, M.S.; Timmerhans, K.D. Plant Design and Economics for Chemical Engineers, Chemical Engineering Series, 3rd ed.; Me Gram-Hill: Tokyo, Japan, 1981. [Google Scholar]
- El-Didamony, H.; Khalil, A.; Ahmed, I.A.; Heikal, M. Preparation of b-dicalcium silicate (b-C2S) and calcium sulfoaluminate C3A3CS phases using non-traditional nano-materials. Constr. Build. Mater. 2012, 35, 77–83. [Google Scholar] [CrossRef]
- Chena, B.; Xisheng, X.; Xiaoming, C.; Lingjun, K.; Diyun, C. Transformation behavior of gibbsite to oxyhydroxide aluminum by steam-assisted, Synthesis. J. Solid State Chem. 2018, 265, 237–243. [Google Scholar] [CrossRef]
- Zapf, J.T.; Fultz, W.C.; Liu, S.T.; Wozniak, M.E. Silica-coated oxyhydroxide aluminum composites suitable for dentifrices. US Pat. 2007, 7, 166–271. [Google Scholar]
- El-Faramawy, I.; Morsy, N.S.Z.; Taher, F.A.; Sabry, R.M.; Ali, H.M. Preparation of conductive polymer nano-composite with chitosan and its application in the removal of hexavalent chromium. Egyptian. J. Chem. 2020, 63, 7. [Google Scholar]
- Sun, C.; Wang, Y. Factors influencing preparation of polyaniline doped with hydrochloric acid. Mater. Sci. Pol. 2014, 32, 521–525. [Google Scholar]
- Mohamed, A.A.; Abeer, A.M.; Dalia, M.S.A.; Gehan, M. The uptake of Eriochrome Black T dye from aqueous solutions utilizing waste activated sludge: Adsorption process optimization using factorial design. EJABF 2018, 44, 179–186. [Google Scholar]
- Ghader, Z.; Mahsa, K.; Yusef, O.; Heshmatollah, N.; Shirin, E.; Mohammad, J.; Rajab, R. Eriochrme black-T removal from aqueous environment by surfactant modified clay: Equilibrium, kinetic, isotherm, and thermodynamic studies. Toxin Rev. 2018, 38, 1–11. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |











| Kinetic Models | Parameter | SCB | SBDP |
|---|---|---|---|
| PFORE | qe, exp(mg g−1) | 160 | 163.8 |
| qe,cal(mg g−1) | 13.5 | 9.2 | |
| Kads(min−1) | 0.0269 | 0.0264 | |
| R2 | 0.9444 | 0.6762 | |
| PSORE | qe, cal(mg g−1) | 163.13 | 165.3 |
| K2(g mg−1 min−1) | 0.0037 | 0.0054 | |
| R2 | 0.9995 | 0.9998 | |
| Mories–Weber | Kd(mg g−1 min0.5) | 0.5299 | 0.7598 |
| R2 | 0.9951 | 0.9885 |
| Kinetic Isotherm | Parameter | SCB | SBDP |
|---|---|---|---|
| Langmuir | qe, exp (mg g−1) | 160 | 163.8 |
| qe,cal(mg g−1) | 168.4 | 258.3 | |
| KL(L mg−1) | 0.0232 | 0.0040 | |
| R2 | 0.9981 | 0.9712 | |
| Freundlich | KF(moln− 1 Ln g−1) | 101.5 | 156.8 |
| n | 6.2 | 14.37 | |
| R2 | 0.9884 | 0.5349 | |
| D-R model | E(kJ mol−1) | 0.711 | 0.714 |
| q(D-R) (mg g−1) | 788.3 | 244.6 | |
| R2 | 0.9993 | 0.9992 |
| Parameter | T (K) | LnKL | ∆Ho (KJ.mol−1) | ∆So (J.mol−1.K−1) | ∆Go (kJ.mol−1) | R2 |
|---|---|---|---|---|---|---|
| Ni+2/SCB | 303 | 7.11 | 8.16 | 85.55 | −17.91 | 0.9998 |
| 313 | 7.3 | −18.99 | ||||
| 323 | 7.4 | −19.87 | ||||
| Ni+2/SBDP | 300 | 8.58 | −83.19 | −202.77 | −21.62 | 0.9968 |
| 313 | 6.77 | −17. 59 | ||||
| 323 | 5.53 | −14.85 |
| Raw Material | Amount | Total Price (USD) |
|---|---|---|
| Al-dross powder | 500 g | No cost (waste) |
| Commercial HCl | 3.00 L | 6.20 |
| Sodium silicate | 1.00 L | 0.01 |
| Aniline | 20 g | 0.06 |
| Sodium per sulphate | 10 g | 0.10 |
| Net cost | 6.3 (USD) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
A. Ahmed, I.; S. Hussein, H.; H. Ragab, A.; S. Al-Radadi, N. Synthesis and Characterization of Silica-Coated Oxyhydroxide Aluminum/Doped Polymer Nanocomposites: A Comparative Study and Its Application as a Sorbent. Molecules 2020, 25, 1520. https://doi.org/10.3390/molecules25071520
A. Ahmed I, S. Hussein H, H. Ragab A, S. Al-Radadi N. Synthesis and Characterization of Silica-Coated Oxyhydroxide Aluminum/Doped Polymer Nanocomposites: A Comparative Study and Its Application as a Sorbent. Molecules. 2020; 25(7):1520. https://doi.org/10.3390/molecules25071520
Chicago/Turabian StyleA. Ahmed, Inas, H. S. Hussein, Ahmed H. Ragab, and Najlaa S. Al-Radadi. 2020. "Synthesis and Characterization of Silica-Coated Oxyhydroxide Aluminum/Doped Polymer Nanocomposites: A Comparative Study and Its Application as a Sorbent" Molecules 25, no. 7: 1520. https://doi.org/10.3390/molecules25071520
APA StyleA. Ahmed, I., S. Hussein, H., H. Ragab, A., & S. Al-Radadi, N. (2020). Synthesis and Characterization of Silica-Coated Oxyhydroxide Aluminum/Doped Polymer Nanocomposites: A Comparative Study and Its Application as a Sorbent. Molecules, 25(7), 1520. https://doi.org/10.3390/molecules25071520