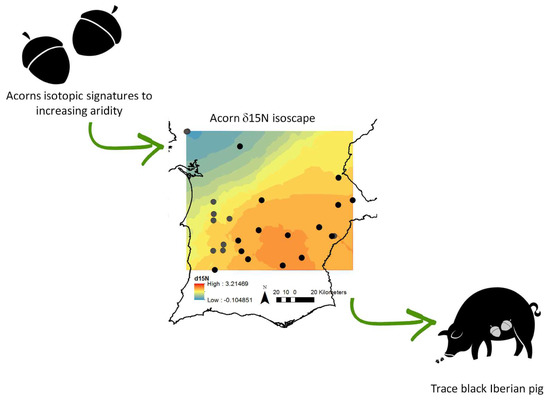
Acorn Isotopic Composition: A New Promising Tool for Authenticity Maps of Montado’s High-Value Food Products
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Study Sites
3.2. Morphological and Physiological Traits
3.3. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferraz-de-Oliveira, M.I.; Azeda, C.; Pinto-Correia, T. Management of Montados and Dehesas for High Nature Value: An interdisciplinary pathway. Agroforest Syst. 2016, 90, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Estévez, V.; García Martínez, A.; Perea Muñoz, J.M.; Mata Moreno, C.; Gómez Castro, A.G. Producción de bellota en la dehesa: Factores influyentes. Arch. Zootec 2007, 56R, 25–43. [Google Scholar]
- Sottomayor, M. Potencial económico da Bolota em Portugal: Análise Exploratória. Presented at the Oral Communication 1st Symposium A Bolota: O futuro de um Alimento com Passado. Frutos Silvestres Comestíveis-Guia Prático, Montemor-o-Novo, Portugal, 20 March 2015; Azevedo, A., Ed.; Quercus Publication: London, UK, 2015. [Google Scholar]
- Pinto-Correia, T. Threatened landscape in Alentejo, Portugal: The ‘montado’and other ‘agro-silvo-pastoral’ systems. Landscape Urban. Plan. 1993, 24, 43–48. [Google Scholar] [CrossRef]
- Bugalho, M.N.; Caldeira, M.C.; Pereira, J.S.; Aronson, J.; Pausas, J.G. Mediterranean cork oak savannas require human use to sustain biodiversity and ecosystem services. Front. Ecol Environ. 2011, 9, 278–286. [Google Scholar] [CrossRef] [Green Version]
- Ministerio de Agricultura Pesca y Alimentación. REAL DECRETO 1469/2007, de 2 de Noviembre, Por el Que se Aprueba la Norma de Calidad Para la Carne, el Jamón, la Paleta y la Caña de Lomo Ibéricos. BOE no. 264, de 3/11/2007; Ministerio de Agricultura, Pesca y Alimentación: Madrid, Spain; pp. 45087–45104.
- Vinha, A.F.; Barreira, J.C.M.; Costa, A.S.G.; Oliveira, M.B.P.P. A new age for Quercus spp. fruits: Review on nutritional and phytochemical composition and related biological activities of acorns. Compr. Rev. Food Sci. Food Saf. 2016, 15, 947–981. [Google Scholar] [CrossRef] [Green Version]
- Cantos, E.; Espín, J.C.; López-Bote, C.; de la Hoz, L.; Ordóñez, J.A.; Tomás-Barberán, F.A. Phenolic compounds and fatty acids from acorns (Quercus spp.), the main dietary constituent of free-ranged Iberian pigs. J. Agric. Food Chem. 2003, 51, 6248–6255. [Google Scholar] [CrossRef]
- Galván, J.; Jorrín Novo, J.J.; Cabrera, A.G.; Ariza, D.; García-Olmo, J.; Cerrillo, R.M.N. Population variability based on the morphometry and chemical composition of the acorn in Holm oak (Quercus ilex subsp. ballota [Desf.] Samp.). Eur. J. For. Res. 2012, 131, 893–904. [Google Scholar] [CrossRef]
- Gea-Izquierdo, G.; Cañellas, I.; Montero, G. Acorn production in Spanish holm oak woodlands. Invest. Agrar: Sist. Recur. For. 2006, 15, 339–354. [Google Scholar] [CrossRef] [Green Version]
- Plieninger, T.; Pulido, F.J.; Konold, W. Effects of land-use history on size structure of holm oak stands in Spanish dehesas: Implications for regeneration and restoration. Environ. Conserv. 2003, 30, 61–70. [Google Scholar] [CrossRef]
- Dawson, T.E.; Mambelli, S.; Plamboek, H.; Templer, P.H.; Tu, K.P. Stable isotopes in plant ecology. Annu. Rev. Ecol. Syst. 2002, 33, 507–559. [Google Scholar] [CrossRef]
- West, J.B.; Kreuzer, H.W.; Ehleringer, J.R. Approaches to plant hydrogen and oxygen isoscapes generation. In Isoscapes, understanding movement, pattern, and process on Earth through isotope mapping; West, J.B., Bowen, G.J., Dawson, T.E., Tu, K.P., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 161–168. [Google Scholar] [CrossRef]
- Barbour, M.M.; Andrews, T.J.; Farquhar, G.D. Correlations between oxygen isotope ratios of wood constituents of Quercus and Pinus samples from around the world. Austr. J. Plant. Physiol. 2001, 28, 335–348. [Google Scholar] [CrossRef]
- Bowen, G.J.; Wassenar, I.I.; Hobson, K.A. Global application of stable hydrogen and oxygen isotopes to wildlife forensics. Oecologia 2005, 143, 337–348. [Google Scholar] [CrossRef]
- Bowen, G.J.; West, J.B.; Vaughn, B.H.; Dawson, T.E.; Ehleringer, J.R.; Fogel, M.L.; Hobson, K.A.; Hoogewerff, J.; Kendall, C.; Lai, C.T.; et al. Isoscapes to address large-scale Earth science challenges. Eos 2009, 90, 109–116. [Google Scholar] [CrossRef] [Green Version]
- West, J.B.; Bowen, G.J.; Ceiling, T.E.; Ehleringer, J.R. Stable isotopes as one of nature’s ecological recorders. Trends Ecol. Evol. 2006, 21, 408–414. [Google Scholar] [CrossRef]
- Bowen, G.J.; West, J.B.; Hoogewerff, J. Isoscapes: Isotope mapping and its applications. J. Geochem. Explor. 2009, 102, 5–7. [Google Scholar] [CrossRef]
- González-Martin, I.; González -Pérez, C.; Hernández Méndez, J.; Marqués-Macias, E.; Sanz Poveda, F. Use of isotope analysis to characterize meat from Iberian-breed swine. Meat Sci. 1999, 52, 437–441. [Google Scholar] [CrossRef]
- González-Martin, I.; González -Pérez, C.; Hernández Méndez, J.; Sánchez González, C. Differentiation of dietary regimene of Iberian swine by means of isotopic analysis of carbon and sulphur in hepatic tissue. Meat. Sci. 2001, 58, 25–30. [Google Scholar] [CrossRef]
- Delgado-Chavero, C.L.; Zapata-Márquez, E.; García-Casco, J.M.; Paredes-Torronteras, A. Statistical model for classifying the feeding systems of Iberian pigs through Gas Chromatography (GC-FID) and Isotope Ratio Mass Spectrometry (GC-C-IRMS). Grasas y Aceites 2013, 64, 157–165. [Google Scholar] [CrossRef] [Green Version]
- Recio, C.; Martín, Q.; Raposo, C. GC-C-IRMS Analysis of FAMEs as a Tool to Ascertain the Diet of Iberian Pigs Used for the Production of Pork Products with High Added Value. Grasas y Aceites 2013, 64, 181–190. [Google Scholar] [CrossRef] [Green Version]
- López-Bascón, M.A.; Priego-Capote, F.; Calderón-Santiago, M.; Sánchez De Medina, V.; Moreno-Rojas, J.M.; García-Casco, J.M.; Luque De Castro, M.D. Determination of Fatty Acids and Stable Carbon Isotopic Ratio in Subcutaneous Fat to Identify the Feeding Regime of Iberian Pigs. J. Agric. Food Chem. 2015, 63, 692–699. [Google Scholar] [CrossRef]
- Merouani, H.; Apolinário, L.M.; Almeida, M.-H.; Pereira, J.S. Morphological and physiological maturation of acorns of cork oak (Quercus suber L.). Seed Sci. Technol. 2003, 31, 111–124. [Google Scholar] [CrossRef]
- Greenberg, C.H. Individual variation in acorn production by five species of Southern Appalachian oaks. Forest Ecol Manag 2000, 132, 199–210. [Google Scholar] [CrossRef]
- Díaz-Fernández, P.M.; Climent, J.; Gil, L. Biennial acorn maturation and its relationship with flowering phenology in Iberian populations of Quercus suber. Trees 2004, 18, 615–621. [Google Scholar] [CrossRef]
- Carbonero, M.D.; Fernández, P.; Bázquez, A.; Navarro, R. Evaluación de la producción y del calibre de bellotas de Quercus ilex L. subsp. ballota (Desf) Samp a lo largo de un ciclo de poda: Resultados de las campañas 2001–2002 y 2002–2003. In XLIII Reunión Científica de la SEEP; Robles, A., Ramos, E., Morales, M.C., De Simón, E., González Rebollar, J.L., Boza, J., Eds.; Junta de Andalucia: Granada, Spain, 2003; pp. 645–650. [Google Scholar]
- Akcan, T.; Gökçe, R.; Asensio, M.; Estévez, M.; Morcuende, D. Acorn (Quercus spp.) as a novel source of oleic acid and tocopherols for livestock and humans: Discrimination of selected species from Mediterranean forest. J. Food Sci. Technol. 2017, 54, 3050–3057. [Google Scholar] [CrossRef]
- Afzal-Rafii, Z.; Dodd, R.S.; Pelleau, Y. Mediterranean evergreen oak diversity: Morphological and chemical variation of acorns. Can. J. Bot 1992, 70, 1459–1466. [Google Scholar] [CrossRef]
- Nunes, A.; Köbel, M.; Pinho, P.; Matos, P.; de Bello, F.; Correia, O.; Branquinho, C. Which plant traits respond to aridity? A critical step to assess functional diversity in Mediterranean drylands. Agric. For. Meteorol 2017, 239, 176–184. [Google Scholar] [CrossRef]
- Wang, C.; Wang, X.; Liu, D.; Wu, H.; Lü, X.; Fang, Y.; Cheng, W.; Luo, W.; Jiang, P.; Shi, J.; et al. Aridity threshold in controlling ecosystem nitrogen cycling in arid and semi-arid grasslands. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [Green Version]
- Delgado-Baquerizo, M.; Maestre, F.T.; Gallardo, A.; Quero, J.L.; Ochoa, V.; García-Gómez, M.; Escolar, C.; García-Palacios, P.; Berdugo, M.; Valencia, E.; et al. Aridity modulates N availability in arid and semiarid Mediterranean grasslands. PLoS ONE 2013, 8, e59807. [Google Scholar] [CrossRef] [Green Version]
- Austin, A.T.; Sala, O.E. Carbon nitrogen dynamics across a natural precipitation gradient in Patagonia Argentina. J. Veg. Sci. 2002, 13, 13,351–360. [Google Scholar] [CrossRef]
- Luo, T.; Pan, Y.; Ouyang, H.; Shi, P.; Luo, J.; Yu, Z.; Lu, Q. Leaf area index and net primary productivity along subtropical to alpine gradients in the Tibetan Plateau. Global Ecol Biogeogr 2004, 13, 345–358. [Google Scholar] [CrossRef]
- Austin, A.T.; Vitousek, P.M. Nutrient dynamics on a precipitation gradient in Hawai’i. Oecologia 1998, 113, 519–529. [Google Scholar] [CrossRef]
- Schuur, E.A.G.; Matson, P.A. Net primary productivity and nutrient cycling across a mesic to wet precipitation gradient in Hawaiian montane forest. Oecologia 2001, 128, 431–442. [Google Scholar] [CrossRef]
- Aranibar, J.N.; Otter, L.; Macko, S.A.; Feral, C.J.; Epstein, H.E.; Dowty, P.R.; Eckardt, F.; Shugart, H.H.; Swap, R.J. Nitrogen cycling in the soil–plant system along a precipitation gradient in the Kalahari sands. Global Change Biol. 2004, 10, 359–373. [Google Scholar] [CrossRef]
- McCulley, R.L.; Burke, I.C.; Lauenroth, W.K. Conservation of nitrogen increases with precipitation across a major grassland gradient in the central Great Plains of North America. Oecologia 2009, 159, 571–581. [Google Scholar] [CrossRef]
- Szukics, U.; Abell, G.C.J.; Hold, V.; Mitter, B.; Asessitsch, A.H.; Hackl, E.; Zechmeister-Boltenstern, S. Nitrifiers and denitrifiers respond rapidly to changed moisture and increasing temperature in a pristine forest soil. FEMS Microbiol. Ecol. 2010, 72, 395–406. [Google Scholar] [CrossRef]
- Thiagalingam, K.; Kanehiro, Y. Effect of temperature on nitrogen transformation in Hawaiian soils. Plant. Soil 1973, 38, 177–189. [Google Scholar] [CrossRef]
- Barrett, J.E.; Burke, I.C. Nitrogen retention in semiarid ecosystems across a soil organic-matter gradient. Ecol. Appl. 2002, 12, 878–890. [Google Scholar] [CrossRef]
- Peri, P.L.; Ladd, B.; Pepper, D.S.; Bonser, S.P.; Laffan, S.W.; Amelung, W. Carbon (d13C) and nitrogen (d15N) stable isotope composition in plant and soil in Southern Patagonia’s native forests. Global Change Biol. 2012, 18, 311–321. [Google Scholar] [CrossRef]
- Kahmen, A.; Wanek, W.; Buchmann, N. Foliar d15N values characterize soil N cycling and reflect nitrate or ammonium preference of plants along a temperate grassland gradient. Oecologia 2008, 156, 861–870. [Google Scholar] [CrossRef] [Green Version]
- Houlton, B.Z.; Sigman, D.M.; Hedin, L.O. Isotopic evidence for large gaseous nitrogen losses from tropical rainforests. PNAS 2006, 103, 8745–8750. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, C.; Maia, R.; Miranda, M.; Ribeirinho, M.; Nogueira, J.M.F.; Máguas, C. Stable isotope analysis for green coffee bean: A possible method for geographic origin discrimination. J. Food Comp. Anal. 2009, 22, 463. [Google Scholar] [CrossRef]
- Trabucco, A.; Zomer, R. Global Aridity Index and Potential Evapotranspiration (ET0) Climate Database v2 Figshare Fileset. 2019. Available online: https://doi.org/10.6084/m9.figshare.7504448.v3.
- StatSoft, Inc. STATISTICA (Data Analysis Software System), Version 8.0. 2007. Available online: https://www.statsoft.com (accessed on 25 February 2020).
- Middleton, N.; Thomas, D. (Eds.) World Atlas of Desertification, UNEP; Edward Arnold: London, UK, 1992; pp. 1–69. [Google Scholar]
Sample Availability: Biological samples are not available from the authors. The data that support the findings of this study are available upon reasonable request from the authors and soon will be submitted to REALMed database for public access. |





| Morphological Trait | Quercus rotundifolia (QR) | Quercus suber (QS) |
|---|---|---|
| Weight (g) | 5.64 ± 1.21 (3.91–7.48) | 6.18 ± 2.45 (3.29–10.18) |
| Length (mm) | 37.57 ± 3.24 (33.39–43.50) | 33.80 ± 5.88 (25.33–44.51) |
| Diameter (mm) | 14.75 ± 1.59 (11.76–16.85) | 15.49 ± 2.50 (11.93–18.96) |
| Volume (cm3) | 4.34 ± 1.06 (2.58–6.03) | 4.51 ± 2.05 (2.16–8.11) |
| Weight | Length | Diameter | Volume | AI | |||
|---|---|---|---|---|---|---|---|
| Weight | 1.00 | 0.69 ** | 0.75 ** | 0.91 *** | −0.19 | QR | |
| Length | QS | 0.93 *** | 1.00 | 0.17 | 0.47 | −0.17 | |
| Diameter | 0.90 *** | 0.74* | 1.00 | 0.94 *** | −0.08 | ||
| Volume | 0.98 *** | 0.91 *** | 0.93 *** | 1.00 | −0.14 | ||
| AI | −0.57 * | −0.62 * | −0.32 | −0.54 | 1.00 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alegria, C.; Antunes, C.; Giovanetti, M.; Abreu, M.; Máguas, C. Acorn Isotopic Composition: A New Promising Tool for Authenticity Maps of Montado’s High-Value Food Products. Molecules 2020, 25, 1535. https://doi.org/10.3390/molecules25071535
Alegria C, Antunes C, Giovanetti M, Abreu M, Máguas C. Acorn Isotopic Composition: A New Promising Tool for Authenticity Maps of Montado’s High-Value Food Products. Molecules. 2020; 25(7):1535. https://doi.org/10.3390/molecules25071535
Chicago/Turabian StyleAlegria, Carla, Cristina Antunes, Manuela Giovanetti, Marta Abreu, and Cristina Máguas. 2020. "Acorn Isotopic Composition: A New Promising Tool for Authenticity Maps of Montado’s High-Value Food Products" Molecules 25, no. 7: 1535. https://doi.org/10.3390/molecules25071535
APA StyleAlegria, C., Antunes, C., Giovanetti, M., Abreu, M., & Máguas, C. (2020). Acorn Isotopic Composition: A New Promising Tool for Authenticity Maps of Montado’s High-Value Food Products. Molecules, 25(7), 1535. https://doi.org/10.3390/molecules25071535